CMC Technical Writing: A Critical Enabler During Product Development
The Windsor Framework: Procedural Impact

13 Jun, 2025
This is the second blog in the Windsor Framework series (the first blog can be read here). This blog will discuss the procedural impact of the changes introduced under the Windsor Framework.
Reference Medicinal Products and Equivalence Testing
Since January 1, 2025, a UK Reference Medicinal Product (RMP) is needed for all UK-wide MAAs for generic, hybrid or biosimilar applications. It is not acceptable to make use of a European Reference Product (ERP), except for pending applications for which the procedure started before January 1, 2025.
For bioequivalence or therapeutic equivalence testing, the comparator product does not have to be the UK RMP sourced from UK market but must be representative of the UK RMP. Generally, the comparator product should be sourced from the UK, but if not, the applicant should provide evidence that it is representative of the UK RMP. In case there is no UK reference product because the active substance had never been authorized in the UK, then the application will be classified as a new active substance. This is not different from the current situation, where MHRA classifies a product that is authorized in Europe, but is new to the UK, as a new active substance. Products with an NI-only MA will continue to reside under the EU requirements after January 1, 2025. Union authorizations (i.e. centrally authorized product authorizations) that were not grandfathered at time of EU exit cannot be used as RMPs going forward. All these requirements apply to applications, whether they are made directly as a national application or through the International Recognition Procedure. Data and Market Exclusivity periods (DME) will be those applicable in the UK. For grandfathered EU MAs, the start dates that were established at the time of EU authorization are respected.
These requirements apply to both Category 1 and Category 2 products. Specific guidance is available on reference products for biosimilar products. If the necessary data or information is not available, an alternative legal basis for the submission is needed. This includes well-established use under regulation 54 of the Human Medicines Regulations (HMRs), or mixed dossier under regulation 50 of HMRs.
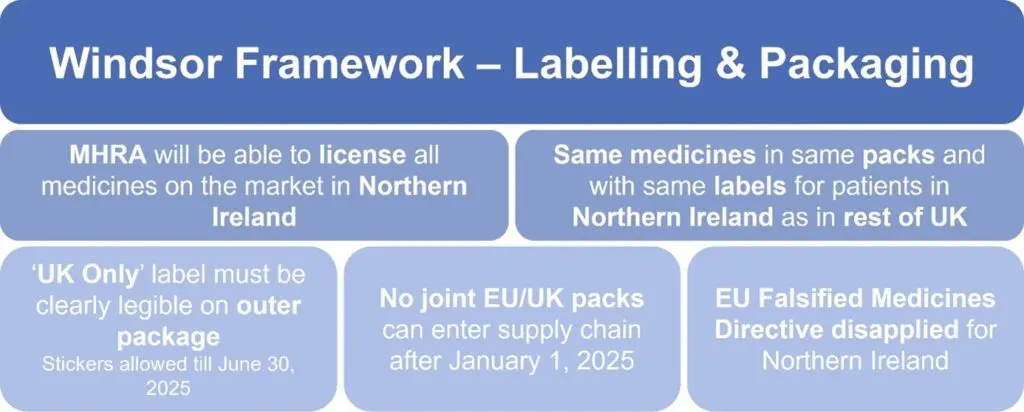
New Labelling & Packaging Requirements
All medicines released in the UK from January 1, 2025, must prominently display the ‘UK Only’ label on the outer packaging. While stickers can be used as a temporary measure, they must be replaced with permanent outer packaging by June 30, 2025. This requirement applies across all prescription, pharmacy and general sale medicines, but not for APIs or excipients.
With the implementation of the Windsor Framework, the EU’s FMD will no longer apply in Northern Ireland. Elements of the EU FMD packaging, labelling and barcode requirements for medicines must not appear on medicines being supplied to the UK. However, companies can continue to use 2D barcodes and serialization numbers in line with published guidance, as long as these are not recognized by the EU repositories system. Furthermore, the use of anti-tamper devices is also encouraged.
To facilitate a smooth transition, the MHRA has outlined flexible approaches. Existing packs placed on the market before January 1, 2025, can remain available until their expiry date. Companies can notify MHRA of artwork updates through self-certification or as part of a broader regulatory submission. Joint UK-EU packs will no longer be allowed after January 1, 2025, though already-released stock can continue circulating.
The presence of ‘UK Only’ on the packaging does not mean packs cannot be exported from the UK. MHRA engaged with other regulators to explain this and will continue to engage on this matter as necessary.
If there are differences in product information between the marketing authorization (MA) of Great Britain and the corresponding EU MA, this should be managed on a case-by-case basis. MHRA is aware that there may be some differences, and that this could be both scientific and technical, but also administrative. Therefore, when considering that there may need to be updates to internal documents, processes, systems, following on from the 1st of January 2025 expansion of the GB SmPC to UK-wide, the MHRA will take a pragmatic approach and allow a reasonable time for these changes to be made. MA holders only need to communicate differences between the GB and EU product information, where this results in a change of clinical practice for healthcare professionals in Northern Ireland which affects patient safety. For this, the current advice for Direct to Healthcare Professional Communication (DHPCs) should be followed.
Figure 1: Main changes for labelling and packaging requirements under the Windsor Framework
Impact on Distribution and Manufacturing
Since January 2025, Northern Ireland-based manufacturers will no longer need Responsible Person (import) (RPi) oversight when supplying medicines to Great Britain. However, medicines moving between the UK and the EU will face stricter separation. Once placed on the UK market, medicines cannot be stored in the EU for further distribution, reducing previous flexibility of some companies.
Compliance teams will begin inspecting firms against the new Windsor Framework rules from January 1, 2025, ensuring adherence to packaging, licensing, and distribution requirements.
Advertising and Promotion of medicines
As a result of the Windsor Framework, the vast majority of marketing authorizations will be UK-wide and as such can be advertised UK-wide (refer to Part 14 (Advertising) Human Medicines Regulations 2012). The only exceptions are cases where a medicinal product is only licensed in part of the UK, such as products with a Northern Ireland only license. These products can therefore only be advertised in Northern Ireland. Additional information elements may continue to be required in these cases, as currently outlined in the MHRA Blue Guide.
Professional samples to Persons Qualified to Prescribe or Supply medicines shall be no larger than the smallest presentation available for sale in the UK or part of the UK where supply is made. All other requirements remain unchanged under the Windsor Framework.
Previously, UK-wide advertisements may list a PLGB number for GB and an EU number for Northern Ireland. From January 1, 2025, the PLGB number will cover these UK-wide advertisements, while the EU license number does not apply anymore to the territory of Northern Ireland. When making any updates to Prescribing Information, companies and advertisers are advised to take the opportunity to review the information to remove, amend, or clarify any references to territories as may be appropriate to avoid any potential confusion. In line with requirements for keeping information up to date, material that no longer complies should be withdrawn from use.
In case there are different product indications between UK and other European territories, joint advertising across the continent is not possible, as this would not comply with UK SPC. Different safety information between UK and other European territories does not necessarily preclude Europe-wide advertising, but care will be required to ensure the safety of UK public health is not in any way compromised.
Final Considerations for Pharma Compliance Under the Windsor Framework
The Windsor Framework significantly streamlines medicine regulation in Northern Ireland by implementing UK-rules for the development of generics, clear packaging, distribution, manufacturing and Ad Promo activities. The pharma industry must ensure compliance with the new requirements as of January 2025.
For detailed guidance, visit the official MHRA Windsor Framework collection: MHRA Windsor Framework Guidance.
The next, and final, blog will discuss some procedures that are minimally impacted by changes introduced by the Windsor Framework.
Celegence provides pharmaceutical companies with expert guidance on UK regulatory procedures – including reference product strategy, labelling compliance, and marketing submissions. Reach out to us at info@celegence.com to explore how we can support your regulatory goals under the Windsor Framework.
Other Related Articles

11 Nov, 2025

04 Nov, 2025

21 Oct, 2025

08 Oct, 2025

24 Sep, 2025