Common Challenges in DMF Submissions and How to Avoid Them
Expedited Pathways
Designation submissions, regulatory procedures, and maintenance of fast-track approvals.
A clear regulatory strategy is essential for the successful and timely commercialization of medicinal products. Celegence provides tailored regulatory consulting to help pharmaceutical and biotech companies define, execute, and optimize their regulatory pathways—ensuring compliance with global health authorities such as the FDA, EMA, MHRA, and Health Canada.
With a scientific and pragmatic approach, we guide companies in aligning their regulatory solutions with product innovation and patient benefit. From early-stage clinical trial applications to marketing authorization and lifecycle management, our experts provide strategic and hands-on procedural support to help companies bring innovative therapies to market faster.
| Regulatory Strategy & Pathway Planning | Identifying the most efficient filing strategy (e.g., expedited, reliance pathways, centralized, mutual-recognition, or national procedures). |
| Clinical Trial Applications (CTAs) | Preparation and submission of US INDs, EU CTAs, and global clinical trial approvals. |
| Orphan Drug Designation (ODD) & Pediatric Investigation Plans (PIPs) | Preparation, submission, and maintenance of ODD and PIP applications, including transfers. |
| Orphan Drug Designation (ODD) & Pediatric Investigation Plans (PIPs) | Preparation, submission, and maintenance of ODD and PIP applications, including transfers. |
| Scientific Advice & Pre-Submission Meetings | Support for briefing packages and regulatory agency meetings to optimize submission success. |
| Marketing Authorization Applications (MAAs) & Post-Approval Support | Preparing and filing for MAAs, follow-up submissions, and product maintenance. |
| Regulatory Intelligence & Compliance Monitoring | Continuous tracking of global regulatory updates, compliance trends, and strategic adjustments. |
| Lifecycle Management of Module 3 (CMC Documentation) | Ensuring Chemistry, Manufacturing, and Controls (CMC) content is maintained, updated, and aligned with post-authorization requirements. |
| Regulatory Information Management (RIM) & System Set-Up | Support with SPOR, IRIS, XEVMPD, CTIS, PML, EMA service desk, and EMA account management. |
Up to 50% reduction in submission preparation time.
Proven success in FDA, EMA, MHRA, and Health Canada submissions.
Providing regulatory consulting for pharmaceutical, biotech, and medical device manufacturers worldwide.
Specializing in oncology, rare diseases, cardiovascular, neurology, and biologics.
Providing regulatory consulting for pharmaceutical, biotech, and medical device manufacturers worldwide.
Analysis of regulatory changes and strategic advice on their impact.
Delivery of regulatory updates via newsletters, blogs, and industry insights.
Country-specific regulatory comparisons and training sessions.
Our proprietary cloud-based dossier management solution eliminates manual steps in the exchange of regulatory dossiers. It allows you to share, view, and review eCTD, NeeS, and other dossier formats from any region and access them in any location.
The hybrid solution offers you cloud-based software as a service whilst keeping your data privately stored on-site or in a virtual private cloud.
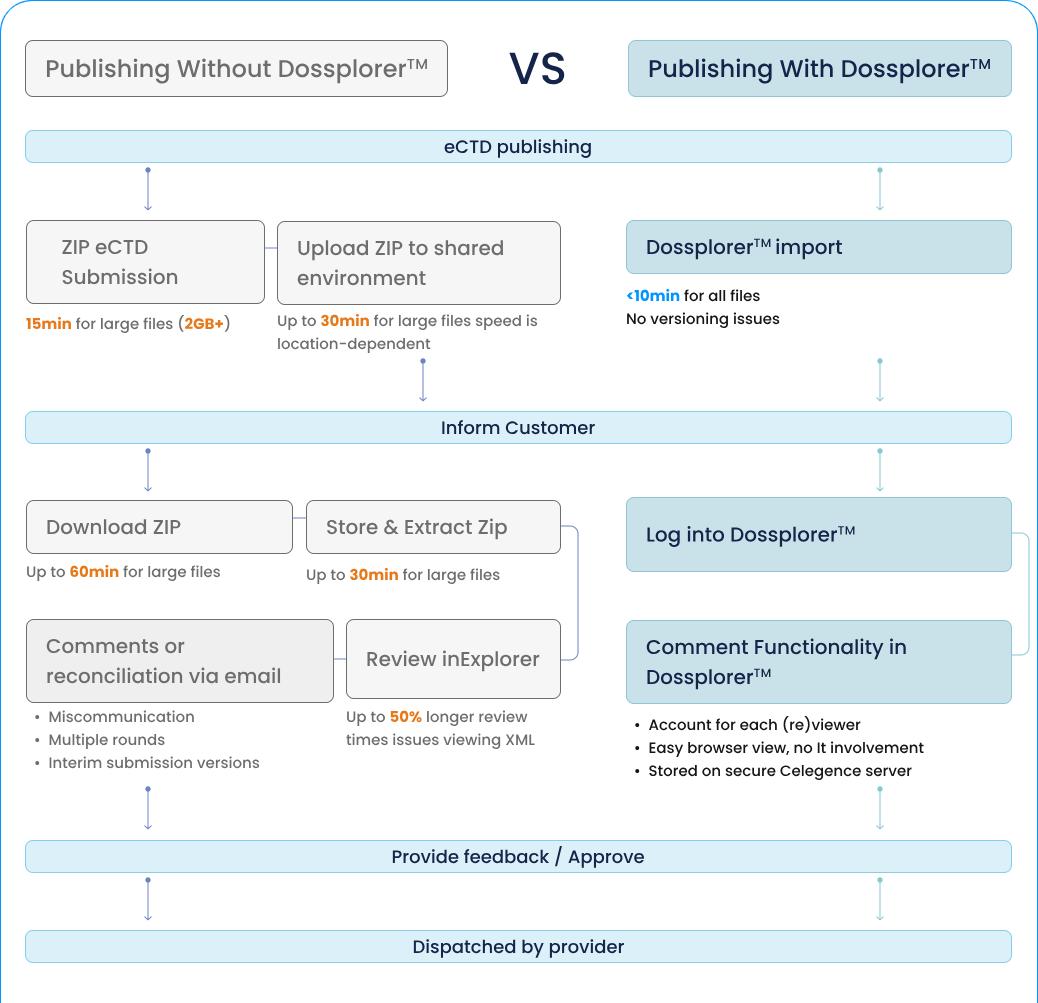
DosscriberTM helps stand-alone regulatory documents for, amongst others, NDA/BLA, IND, NDS, MAA, CTA, PIP, scientific advice, and briefing packages.
With consistent document structure, naming, and eCTD-readiness, Dosscriber™ templates facilitate repurposing documents across countries, products, and dossier types – without rework.
Based in the Netherlands, Maurice has over 27 years’ experience in the pharmaceutical and biotech industry, mainly supporting pre-approval drugs and biologics for oncology, pulmonary and cardiovascular indications, with extensive experience in developing regulatory strategies, leading cross-functional teams in health authority interactions (Scientific Advice, both on national and EU level) regulatory intelligence and applications including document development to support applications such as MAAs, CTAs, EU & US Orphan Drug Applications, PIPs, iPSPs, IND Annual Reports and DSURs. He holds a Master’s degree in Medical Biology from the University of Utrecht and a Doctorate in Medicines from the University of Leiden, Netherlands
Read More
Read Less
Marloes provides regulatory affairs consultancy and services to both big- and small-sized pharmaceutical companies, covering a wide variety of products.
Marloes holds a master in Life Sciences (Drug Innovation) from the University of Utrecht, the Netherlands. For nine years she held several positions at Hoffmann-la Roche in Switzerland, including the role of regulatory intelligence manager and regulatory policy lead for the EMEA region. Awarded DIA Leader of Tomorrow in 2016. Helped author the biosimilar guidelines across the globe.
Read More
Read Less
Diede is a Regulatory Affairs Subject Matter Expert. She specializes in clinical trial applications and scientific advice, with a primary focus on regulatory processes within Europe, including European Medicines Agency (EMA) and Clinical Trials Information System (CTIS). With experience in Regulatory Intelligence, eCTD template development, and Module 1 documentation support – including electronic Application Forms (eAF), Product Information, and Risk Management Plan (RMP) updates – Diede brings a thorough understanding of regulatory strategies to her role. She holds a PhD in Hemato-pathology and both a Bachelor’s and Master’s degree in Biomedical Sciences. Her academic expertise, combined with her industry experience, enables her to navigate regulatory landscapes and provide guidance to clients.
Read More
Read Less
27 Jun, 2025
12 Jun, 2025
05 Jun, 2025
29 May, 2025
From document publishing automation to eCTD submissions and beyond, Celegence is your trusted partner for regulatory affairs excellence. Contact us to learn how we can help you achieve your compliance goals efficiently and cost-effectively.
"*" indicates required fields
Please complete the form:
And our team will get back to you soon.