How AI Is Transforming CER Development for Medical Devices & IVDs
How the Integrated Search Workflow in CAPTIS® Is Transforming Literature Reviews for Regulatory Teams

13 Aug, 2025
At Celegence, we know that efficiency, consistency, and traceability are critical when preparing regulatory documents like Clinical Evaluation Reports (CERs). That’s why we’ve adopted and optimized the Integrated Database Search workflow in CAPTIS®, our go-to solution for systematic literature reviews.
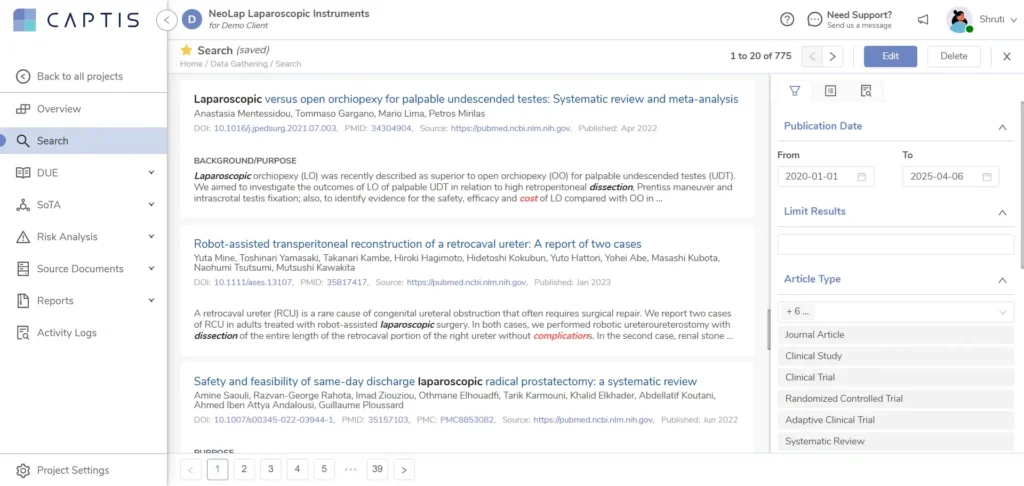
This powerful feature allows our medical writers to search PubMed, Europe PMC, and Google Scholar directly within the platform, replacing the need for manually compiling article data in Excel. By eliminating export-import steps and disconnected tracking, it makes literature reviews significantly faster, cleaner, and fully audit-ready.
These integrated searches were among the first features we prioritized when designing our automated literature workflow, because anyone who has reviewed from spreadsheets knows how time-consuming it is to collate article data, especially from sources like Google Scholar. In fact, many manufacturers still outsource this step because of how tedious and error-prone it can be.
What Makes It So Effective?
-
All-in-One Search Access
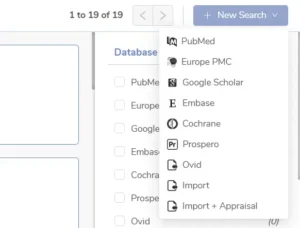
Our writers can search across major scientific databases (PubMed, EU PMC, Google Scholar) without leaving the platform. That means fewer clicks, less toggling, and no time wasted.
-
Smart Review Alignment
Each search can be clearly tagged as part of either the DUE (Device Under Evaluation) or SOTA (State of the Art) workflow. This ensures that literature stays aligned with project goals and regulatory requirements from the start.
-
Built-in Relevance Indicators
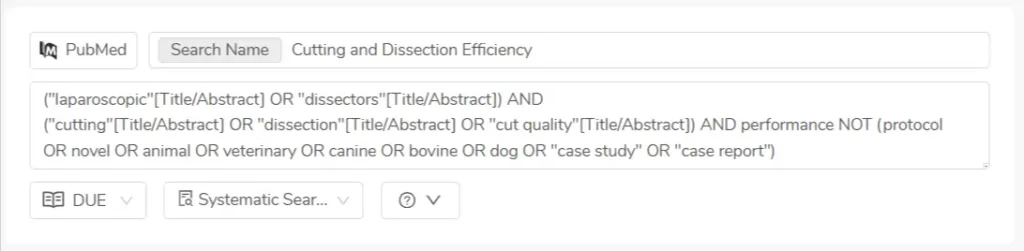
To quickly assess search quality, our teams use positive and negative keywords—a simple but powerful way to check if a search is pulling in the right kind of articles. It helps us know instantly whether a query needs fine-tuning, without wasting review time.
-
Audit-Ready Traceability
Naming searches and maintaining search categories ensures long-term traceability — a huge benefit during regulatory audits or CER updates 2–5 years down the line. This also helps teams align on the overall literature review objectives in the project.
-
Search Editing Without Rework
Need to tweak your search terms or expand a date range mid-project? No problem. You can edit a saved search, rerun it, and retain all previous screening decisions for overlapping articles. No duplication. No data loss.
-
Auto-Update for Long-Term Projects
With Auto-Update enabled, the system will automatically refresh your search results weekly or monthly, capturing new publications as they appear — ideal for projects with rolling deadlines or submissions scheduled months ahead.
-
Rapid Reuse Across Projects
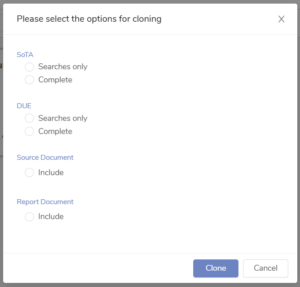
Project cloning combined with search editing lets our team quickly reuse earlier searches — whether it’s extending the date range for a maintenance CER, swapping out key terms to adapt the search for a different device, or reusing the same SOTA strategy across multiple devices. It saves time, ensures consistency, and reduces rework across projects.
“We’ve seen measurable time savings and a more focused authoring and review process since adopting this workflow. Our literature teams are not only working faster and more efficiently, but their output is also far less error-prone and more consistently aligned with regulatory expectations. This ultimately strengthens the quality and reliability of our submissions.”
Ready to Work Smarter With Literature Reviews?
This is just one example of how we’ve optimized regulatory work using CAPTIS®. If your teams handle CERs, PERs, or other evidence-based submissions, the right technology can dramatically simplify your workflows.
Get in touch @ info@celegence.com to see how we can help you scale literature reviews without scaling your headcount.
Other Related Articles

08 Jan, 2026

23 Dec, 2025

08 Dec, 2025

20 Nov, 2025

11 Nov, 2025