EU MDR Compliance Support for a Manufacturer of Ultrasonography (ISG) Covers
Regulatory Consultation & Documentation for Blood Flow Measurement Meters
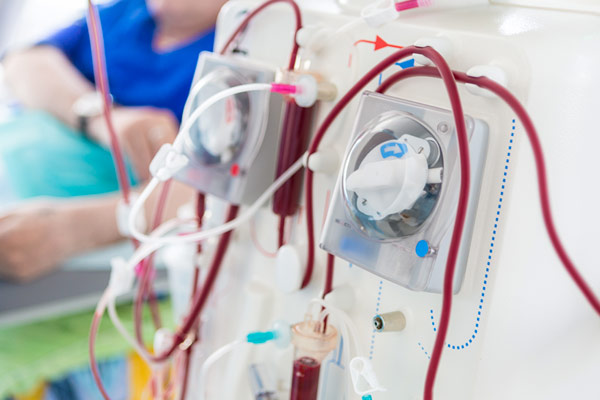
Project Summary
A Manufacturer of Flow measurement meters primarily used for flow measurement of blood during dialysis and other therapies. The Manufacturer has a series of Probes, Sensors and meters based on the applications that belong to Class IIa and III. The accessories belonged to Class I. Products have been in the US and EU market for decades and are MDD Certified.
The Manufacturer initially approached Celegence for CER updates, later extended request to support end to end consultation and documentation for EU MDR approval. The project scope included remediation of QMS, PMS, & Technical documentations, identification of Notified body (NB) and registering review request with finalized NB.
Celegence Solution & Approach:
Celegence performed/ supported following activities:
Highlights:
Gap Analysis on current documentation as per latest ISO Standards involving software, cybersecurity, usability, and electromechanical standards in compliance to ISO 10993, ISO 15223, IEC 60601, IEC 62304, ISO 14971 etc.
Creation and updating of regulatory documents like Technical File, GSPR, Software compliance reports as per IEC 62304, Labeling evaluation reports etc.
Creation of Clinical and PMS documents as per EU MDR like Claims matrix, CEP, LSR, CER, PMS plans & reports, PMCF Plans & reports and SSCP
Finally, the binder was prepared as per the notified body checklist and uploaded to NB portal
Project Success
Celegence successfully completed NB submission by involving expert Clinical writers, Regulatory SMEs, Software and Usability consultants. The key aspects of the project involved: 1. Gap Analysis of four (4) products individually against eleven (11) Harmonized ISO Standards 2. Developed templates for various regulatory documentations 3. Created an automated customer specific Label Specification spreadsheet 4. Extensive Technical Files and General Performance and Safety Requirements checklist for four (4) Product groups 5. Analysis of Sales, Complaints, CAPA and Risk data for the recent reporting period, perform necessary mapping and create reports to support Clinical/ PMS documents.
Get in touch today
to discuss your regulatory needs by reaching out to info@celegence.com or contact us online.
Learn MoreOther Related Articles
18 Jul, 2025
10 Jul, 2025
Contact Us Today
From document publishing automation to eCTD submissions and beyond, Celegence is your trusted partner for regulatory affairs excellence. Contact us to learn how we can help you achieve your compliance goals efficiently and cost-effectively.
"*" indicates required fields






