[fusion_builder_container hundred_percent=”no” hundred_percent_height=”no” hundred_percent_height_scroll=”no” hundred_percent_height_center_content=”yes” equal_height_columns=”no” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” status=”published” border_style=”solid” margin_top=”0″ padding_top=”0px” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ background_blend_mode=”none” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” filter_hue=”0″ filter_saturation=”100″ filter_brightness=”100″ filter_contrast=”100″ filter_invert=”0″ filter_sepia=”0″ filter_opacity=”100″ filter_blur=”0″ filter_hue_hover=”0″ filter_saturation_hover=”100″ filter_brightness_hover=”100″ filter_contrast_hover=”100″ filter_invert_hover=”0″ filter_sepia_hover=”0″ filter_opacity_hover=”100″ filter_blur_hover=”0″ type=”legacy” admin_toggled=”no”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ spacing=”yes” center_content=”no” target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” hover_type=”none” border_style=”solid” border_position=”all” box_shadow=”no” box_shadow_blur=”0″ box_shadow_spread=”0″ padding_top=”0″ margin_top=”0px” margin_bottom=”0px” background_type=”single” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ background_position=”left top” background_repeat=”no-repeat” background_blend_mode=”none” animation_direction=”left” animation_speed=”0.3″ filter_type=”regular” filter_hue=”0″ filter_saturation=”100″ filter_brightness=”100″ filter_contrast=”100″ filter_invert=”0″ filter_sepia=”0″ filter_opacity=”100″ filter_blur=”0″ filter_hue_hover=”0″ filter_saturation_hover=”100″ filter_brightness_hover=”100″ filter_contrast_hover=”100″ filter_invert_hover=”0″ filter_sepia_hover=”0″ filter_opacity_hover=”100″ filter_blur_hover=”0″ last=”true” border_sizes_top=”0″ border_sizes_bottom=”0″ border_sizes_left=”0″ border_sizes_right=”0″ first=”true” min_height=”” link=””][fusion_separator style_type=”none” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” top_margin=”30px” alignment=”center” /][fusion_imageframe image_id=”23262|full” aspect_ratio=”” custom_aspect_ratio=”100″ aspect_ratio_position=”” skip_lazy_load=”” lightbox=”no” gallery_id=”” lightbox_image=”” lightbox_image_id=”” alt=”Accelerate EU MDR Clinical Evidence Generation with CAPTIS®” link=”” linktarget=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” max_width=”” sticky_max_width=”” align_medium=”none” align_small=”none” align=”none” mask=”” custom_mask=”” mask_size=”” mask_custom_size=”” mask_position=”” mask_custom_position=”” mask_repeat=”” style_type=”” blur=”” stylecolor=”” hue=”” saturation=”” lightness=”” alpha=”” hover_type=”none” magnify_full_img=”” magnify_duration=”120″ scroll_height=”100″ scroll_speed=”1″ margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” bordersize=”” bordercolor=”” borderradius=”” z_index=”” caption_style=”off” caption_align_medium=”none” caption_align_small=”none” caption_align=”none” caption_title=”” caption_text=”” caption_title_tag=”2″ fusion_font_family_caption_title_font=”” fusion_font_variant_caption_title_font=”” caption_title_size=”” caption_title_line_height=”” caption_title_letter_spacing=”” caption_title_transform=”” caption_title_color=”” caption_background_color=”” fusion_font_family_caption_text_font=”” fusion_font_variant_caption_text_font=”” caption_text_size=”” caption_text_line_height=”” caption_text_letter_spacing=”” caption_text_transform=”” caption_text_color=”” caption_border_color=”” caption_overlay_color=”” caption_margin_top=”” caption_margin_right=”” caption_margin_bottom=”” caption_margin_left=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” filter_hue=”0″ filter_saturation=”100″ filter_brightness=”100″ filter_contrast=”100″ filter_invert=”0″ filter_sepia=”0″ filter_opacity=”100″ filter_blur=”0″ filter_hue_hover=”0″ filter_saturation_hover=”100″ filter_brightness_hover=”100″ filter_contrast_hover=”100″ filter_invert_hover=”0″ filter_sepia_hover=”0″ filter_opacity_hover=”100″ filter_blur_hover=”0″]https://www.celegence.com/wp-content/uploads/2025/05/Accelerate-EU-MDR-Clinical-Evidence-Generation-with-CAPTIS®.jpg[/fusion_imageframe][fusion_separator style_type=”none” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” top_margin=”30px” alignment=”center” /][fusion_title title_type=”text” marquee_direction=”left” marquee_speed=”15000″ rotation_effect=”bounceIn” display_time=”1200″ highlight_effect=”circle” loop_animation=”off” highlight_width=”9″ highlight_top_margin=”0″ before_text=”” rotation_text=”” highlight_text=”” after_text=”” awb-switch-editor-focus=”” title_link=”off” link_url=”” link_target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” content_align_medium=”” content_align_small=”” content_align=”left” size=”1″ animated_font_size=”” fusion_font_family_title_font=”” fusion_font_variant_title_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” hue=”” saturation=”” lightness=”” alpha=”” animated_text_color=”” text_shadow=”no” text_shadow_vertical=”” text_shadow_horizontal=”” text_shadow_blur=”0″ text_shadow_color=”” text_stroke=”no” text_stroke_size=”1″ text_stroke_color=”” text_overflow=”none” margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” margin_top_mobile=”” margin_bottom_mobile=”” gradient_font=”no” gradient_start_color=”” gradient_end_color=”” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ highlight_color=”” style_type=”default” sep_color=”” link_color=”” link_hover_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=””]EU MDR Clinical Evidence: Meeting the Mark for Regulatory Compliance[/fusion_title][fusion_text columns=”” column_min_width=”” column_spacing=”” rule_style=”” rule_size=”” rule_color=”” hue=”” saturation=”” lightness=”” alpha=”” user_select=”” awb-switch-editor-focus=”” content_alignment_medium=”” content_alignment_small=”” content_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” fusion_font_family_text_font=”” fusion_font_variant_text_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” logics=””]
The transition from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) represents a significant shift in the regulatory landscape for medical devices in the European Union, and has led to stricter requirements for clinical evidence. The European Union Medical Device Regulation (EU MDR) requires manufacturers to provide clinical evidence to demonstrate the safety and performance of their medical devices. This evidence is crucial for obtaining and maintaining CE marking, which allows devices to be sold in the EU.
[/fusion_text][fusion_title title_type=”text” marquee_direction=”left” marquee_speed=”15000″ rotation_effect=”bounceIn” display_time=”1200″ highlight_effect=”circle” loop_animation=”off” highlight_width=”9″ highlight_top_margin=”0″ before_text=”” rotation_text=”” highlight_text=”” after_text=”” awb-switch-editor-focus=”” title_link=”off” link_url=”” link_target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” content_align_medium=”” content_align_small=”” content_align=”left” size=”2″ animated_font_size=”” fusion_font_family_title_font=”” fusion_font_variant_title_font=”” font_size=”20px” line_height=”” letter_spacing=”” text_transform=”” text_color=”” hue=”” saturation=”” lightness=”” alpha=”” animated_text_color=”” text_shadow=”no” text_shadow_vertical=”” text_shadow_horizontal=”” text_shadow_blur=”0″ text_shadow_color=”” text_stroke=”no” text_stroke_size=”1″ text_stroke_color=”” text_overflow=”none” margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” margin_top_mobile=”” margin_bottom_mobile=”” gradient_font=”no” gradient_start_color=”” gradient_end_color=”” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ highlight_color=”” style_type=”default” sep_color=”” link_color=”” link_hover_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=””]What Is Clinical Evidence Under EU MDR?[/fusion_title][fusion_text columns=”” column_min_width=”” column_spacing=”” rule_style=”” rule_size=”” rule_color=”” hue=”” saturation=”” lightness=”” alpha=”” user_select=”” awb-switch-editor-focus=”” content_alignment_medium=”” content_alignment_small=”” content_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” fusion_font_family_text_font=”” fusion_font_variant_text_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” logics=””]
As per EUMDR clinical evidence can be defied as clinical evaluation as “a systematic and planned process to continuously generate, collect, analyse and assess the clinical data pertaining to a device in order to verify the safety and performance, including clinical benefits of the device when used as intended by the manufacturer.” This evidence should be specific to the device of interest and should be able to demonstrate the safety and performance of the device.
The data can be from various sources like but not limited to:
- Data from clinical investigations: These can be performed by the manufacturer, mostly for high-risk devices to collect safety and performance data.
- Published Scientific literature: Article or publications in peer reviewed journals can also be used to provide clinical evidence for scientific devices.
- PMS data: These data reflect real world evidence for the device as they are collected once the device is launched in the market.
- AE databases/Registries: These databases provide specific data for the device related to the reported AE or other details also specifically in terms of registries
[/fusion_text][fusion_title title_type=”text” marquee_direction=”left” marquee_speed=”15000″ rotation_effect=”bounceIn” display_time=”1200″ highlight_effect=”circle” loop_animation=”off” highlight_width=”9″ highlight_top_margin=”0″ before_text=”” rotation_text=”” highlight_text=”” after_text=”” awb-switch-editor-focus=”” title_link=”off” link_url=”” link_target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” content_align_medium=”” content_align_small=”” content_align=”left” size=”3″ animated_font_size=”” fusion_font_family_title_font=”” fusion_font_variant_title_font=”” font_size=”20px” line_height=”” letter_spacing=”” text_transform=”” text_color=”” hue=”” saturation=”” lightness=”” alpha=”” animated_text_color=”” text_shadow=”no” text_shadow_vertical=”” text_shadow_horizontal=”” text_shadow_blur=”0″ text_shadow_color=”” text_stroke=”no” text_stroke_size=”1″ text_stroke_color=”” text_overflow=”none” margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” margin_top_mobile=”” margin_bottom_mobile=”” gradient_font=”no” gradient_start_color=”” gradient_end_color=”” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ highlight_color=”” style_type=”default” sep_color=”” link_color=”” link_hover_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=””]
When and Where Is Clinical Evidence Required
[/fusion_title][fusion_text columns=”” column_min_width=”” column_spacing=”” rule_style=”” rule_size=”” rule_color=”” hue=”” saturation=”” lightness=”” alpha=”” user_select=”” awb-switch-editor-focus=”” content_alignment_medium=”” content_alignment_small=”” content_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” fusion_font_family_text_font=”” fusion_font_variant_text_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” logics=””]
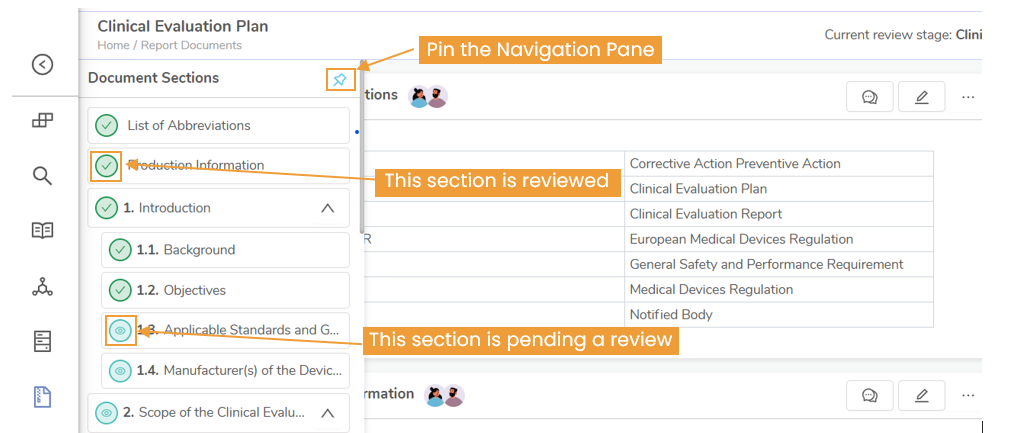
Clinical evidence is required in both premarketing and post marketing phase of device lifecycle in EUMDR. In the premarket phase the manufacturer needs to collect data from clinical investigation and literature to assess the safety and performance of the device. In the post market phase real world clinical evidence can be collected to update the Clinical evaluation documents as necessary. All this data should be used in the CER and should be updated as necessary.
[/fusion_text][fusion_title title_type=”text” marquee_direction=”left” marquee_speed=”15000″ rotation_effect=”bounceIn” display_time=”1200″ highlight_effect=”circle” loop_animation=”off” highlight_width=”9″ highlight_top_margin=”0″ before_text=”” rotation_text=”” highlight_text=”” after_text=”” awb-switch-editor-focus=”” title_link=”off” link_url=”” link_target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” content_align_medium=”” content_align_small=”” content_align=”left” size=”4″ animated_font_size=”” fusion_font_family_title_font=”” fusion_font_variant_title_font=”” font_size=”20px” line_height=”” letter_spacing=”” text_transform=”” text_color=”” hue=”” saturation=”” lightness=”” alpha=”” animated_text_color=”” text_shadow=”no” text_shadow_vertical=”” text_shadow_horizontal=”” text_shadow_blur=”0″ text_shadow_color=”” text_stroke=”no” text_stroke_size=”1″ text_stroke_color=”” text_overflow=”none” margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” margin_top_mobile=”” margin_bottom_mobile=”” gradient_font=”no” gradient_start_color=”” gradient_end_color=”” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ highlight_color=”” style_type=”default” sep_color=”” link_color=”” link_hover_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=””]
What Standards or Thresholds Must Be Met?
[/fusion_title][fusion_text columns=”” column_min_width=”” column_spacing=”” rule_style=”” rule_size=”” rule_color=”” hue=”” saturation=”” lightness=”” alpha=”” user_select=”” awb-switch-editor-focus=”” content_alignment_medium=”” content_alignment_small=”” content_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” fusion_font_family_text_font=”” fusion_font_variant_text_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” logics=””]
To be considered acceptable, clinical evidence must be:
- Device-Specific and Relevant: The data should be specific device and should be aligned to its intended purpose, target patient population, and risk profile. In terms of equivalence the equivalence should be clearly justified clinically, technically and biologically in the CER. Additionally, the data should be sufficient quantitively also.
- Scientifically Robust: Data must be of high-quality, credible, and considered from reliable sources such as clinical investigations, peer-reviewed literature, and structured literature reviews using recognized search methodologies.
- Continuously Updated: Clinical evidence is not static. The CER must be updated regularly, particularly when new risks emerge, performance data changes, or the device is modified. Also based on the device risk there is a review period after which the CER should be revised irrespective of any reasons mentioned before. Additionally, there should be ongoing surveillance for post marketing data for the devices.
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”no” hundred_percent_height=”no” hundred_percent_height_scroll=”no” hundred_percent_height_center_content=”yes” equal_height_columns=”no” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” status=”published” border_style=”solid” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ background_blend_mode=”none” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” filter_hue=”0″ filter_saturation=”100″ filter_brightness=”100″ filter_contrast=”100″ filter_invert=”0″ filter_sepia=”0″ filter_opacity=”100″ filter_blur=”0″ filter_hue_hover=”0″ filter_saturation_hover=”100″ filter_brightness_hover=”100″ filter_contrast_hover=”100″ filter_invert_hover=”0″ filter_sepia_hover=”0″ filter_opacity_hover=”100″ filter_blur_hover=”0″ admin_toggled=”no” type=”legacy”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”2_3″ center_content=”no” target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” hover_type=”none” border_style=”solid” border_position=”all” box_shadow=”no” box_shadow_blur=”0″ box_shadow_spread=”0″ background_type=”single” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ background_position=”left top” background_repeat=”no-repeat” background_blend_mode=”none” animation_direction=”left” animation_speed=”0.3″ filter_type=”regular” filter_hue=”0″ filter_saturation=”100″ filter_brightness=”100″ filter_contrast=”100″ filter_invert=”0″ filter_sepia=”0″ filter_opacity=”100″ filter_blur=”0″ filter_hue_hover=”0″ filter_saturation_hover=”100″ filter_brightness_hover=”100″ filter_contrast_hover=”100″ filter_invert_hover=”0″ filter_sepia_hover=”0″ filter_opacity_hover=”100″ filter_blur_hover=”0″ last=”true” border_sizes_top=”0″ border_sizes_bottom=”0″ border_sizes_left=”0″ border_sizes_right=”0″ first=”true” min_height=”” link=””][fusion_title title_type=”text” marquee_direction=”left” marquee_speed=”15000″ rotation_effect=”bounceIn” display_time=”1200″ highlight_effect=”circle” loop_animation=”off” highlight_width=”9″ highlight_top_margin=”0″ before_text=”” rotation_text=”” highlight_text=”” after_text=”” awb-switch-editor-focus=”” title_link=”off” link_url=”” link_target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” content_align_medium=”” content_align_small=”” content_align=”left” size=”4″ animated_font_size=”” fusion_font_family_title_font=”” fusion_font_variant_title_font=”” font_size=”20px” line_height=”” letter_spacing=”” text_transform=”” text_color=”” hue=”” saturation=”” lightness=”” alpha=”” animated_text_color=”” text_shadow=”no” text_shadow_vertical=”” text_shadow_horizontal=”” text_shadow_blur=”0″ text_shadow_color=”” text_stroke=”no” text_stroke_size=”1″ text_stroke_color=”” text_overflow=”none” margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” margin_top_mobile=”” margin_bottom_mobile=”” gradient_font=”no” gradient_start_color=”” gradient_end_color=”” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ highlight_color=”” style_type=”default” sep_color=”” link_color=”” link_hover_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=””]
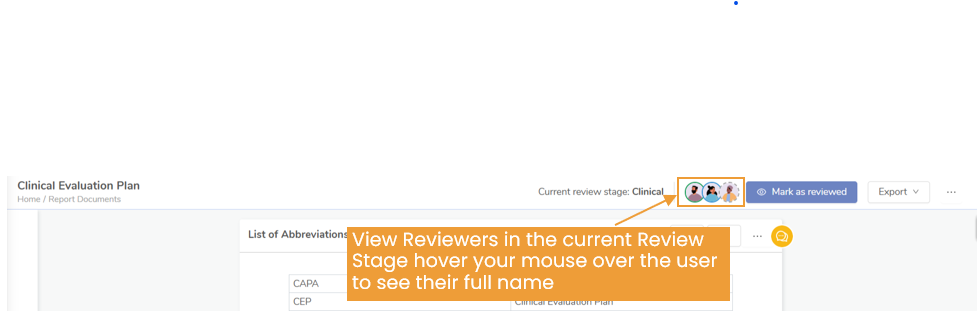
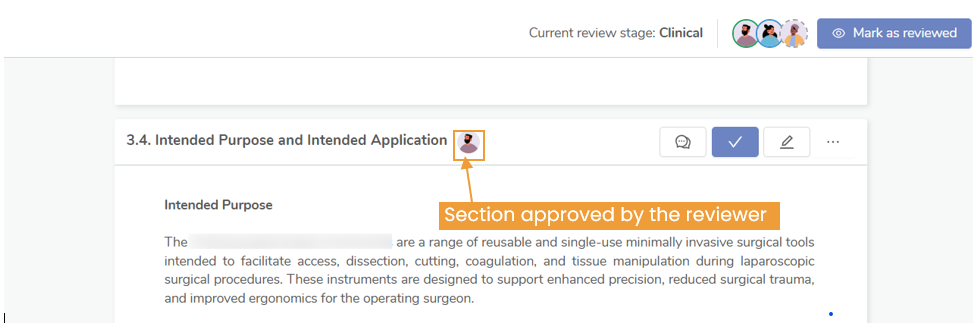
Who Verifies EU MDR Clinical Evidence?
The verification of clinical evidence is primarily conducted by Notified Bodies. These are organizations designated by EU member states to assess the conformity of medical devices with the MDR requirements. The review can be of clinical evaluation or technical documentation, on site review or even PMS audits.
The bar for clinical evaluation has risen significantly, and these bodies are increasingly strict about the sufficiency, traceability, and quality of the data.
Common Pitfalls in Clinical Evidence Preparation
[/fusion_title][fusion_text columns=”” column_min_width=”” column_spacing=”” rule_style=”” rule_size=”” rule_color=”” hue=”” saturation=”” lightness=”” alpha=”” user_select=”” awb-switch-editor-focus=”” content_alignment_medium=”” content_alignment_small=”” content_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” fusion_font_family_text_font=”” fusion_font_variant_text_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” logics=””]
The most common problem currently is the lack of proper clinical evidence to support safety and performance of the device. Another major challenge which is being currently faced by manufacturers is the lack of proper clinical investigation planning. Failing to implement a robust PMS plan or specific PMCF plan also leads to lack of clinical data for the device. Finally, inconsistent documentation, the technical document for a device should align in term of all internal information like intended purpose, claims, warnings precautions etc.
[/fusion_text][fusion_separator style_type=”none” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” top_margin=”30px” alignment=”center” /][fusion_imageframe image_id=”23267|full” aspect_ratio=”” custom_aspect_ratio=”100″ aspect_ratio_position=”” skip_lazy_load=”” lightbox=”no” gallery_id=”” lightbox_image=”” lightbox_image_id=”” alt=”Accelerate Clinical Evidence with CAPTIS®” link=”” linktarget=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” max_width=”” sticky_max_width=”” align_medium=”none” align_small=”none” align=”none” mask=”” custom_mask=”” mask_size=”” mask_custom_size=”” mask_position=”” mask_custom_position=”” mask_repeat=”” style_type=”” blur=”” stylecolor=”” hue=”” saturation=”” lightness=”” alpha=”” hover_type=”none” magnify_full_img=”” magnify_duration=”120″ scroll_height=”100″ scroll_speed=”1″ margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” bordersize=”” bordercolor=”” borderradius=”” z_index=”” caption_style=”off” caption_align_medium=”none” caption_align_small=”none” caption_align=”none” caption_title=”” caption_text=”” caption_title_tag=”2″ fusion_font_family_caption_title_font=”” fusion_font_variant_caption_title_font=”” caption_title_size=”” caption_title_line_height=”” caption_title_letter_spacing=”” caption_title_transform=”” caption_title_color=”” caption_background_color=”” fusion_font_family_caption_text_font=”” fusion_font_variant_caption_text_font=”” caption_text_size=”” caption_text_line_height=”” caption_text_letter_spacing=”” caption_text_transform=”” caption_text_color=”” caption_border_color=”” caption_overlay_color=”” caption_margin_top=”” caption_margin_right=”” caption_margin_bottom=”” caption_margin_left=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” filter_hue=”0″ filter_saturation=”100″ filter_brightness=”100″ filter_contrast=”100″ filter_invert=”0″ filter_sepia=”0″ filter_opacity=”100″ filter_blur=”0″ filter_hue_hover=”0″ filter_saturation_hover=”100″ filter_brightness_hover=”100″ filter_contrast_hover=”100″ filter_invert_hover=”0″ filter_sepia_hover=”0″ filter_opacity_hover=”100″ filter_blur_hover=”0″]https://www.celegence.com/wp-content/uploads/2025/05/Accelerate-Clinical-Evidence-with-CAPTIS®.jpg[/fusion_imageframe][fusion_separator style_type=”none” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” top_margin=”30px” alignment=”center” /][fusion_title title_type=”text” marquee_direction=”left” marquee_speed=”15000″ rotation_effect=”bounceIn” display_time=”1200″ highlight_effect=”circle” loop_animation=”off” highlight_width=”9″ highlight_top_margin=”0″ before_text=”” rotation_text=”” highlight_text=”” after_text=”” awb-switch-editor-focus=”” title_link=”off” link_url=”” link_target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” content_align_medium=”” content_align_small=”” content_align=”left” size=”4″ animated_font_size=”” fusion_font_family_title_font=”” fusion_font_variant_title_font=”” font_size=”20px” line_height=”” letter_spacing=”” text_transform=”” text_color=”” hue=”” saturation=”” lightness=”” alpha=”” animated_text_color=”” text_shadow=”no” text_shadow_vertical=”” text_shadow_horizontal=”” text_shadow_blur=”0″ text_shadow_color=”” text_stroke=”no” text_stroke_size=”1″ text_stroke_color=”” text_overflow=”none” margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” margin_top_mobile=”” margin_bottom_mobile=”” gradient_font=”no” gradient_start_color=”” gradient_end_color=”” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ highlight_color=”” style_type=”default” sep_color=”” link_color=”” link_hover_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=””]
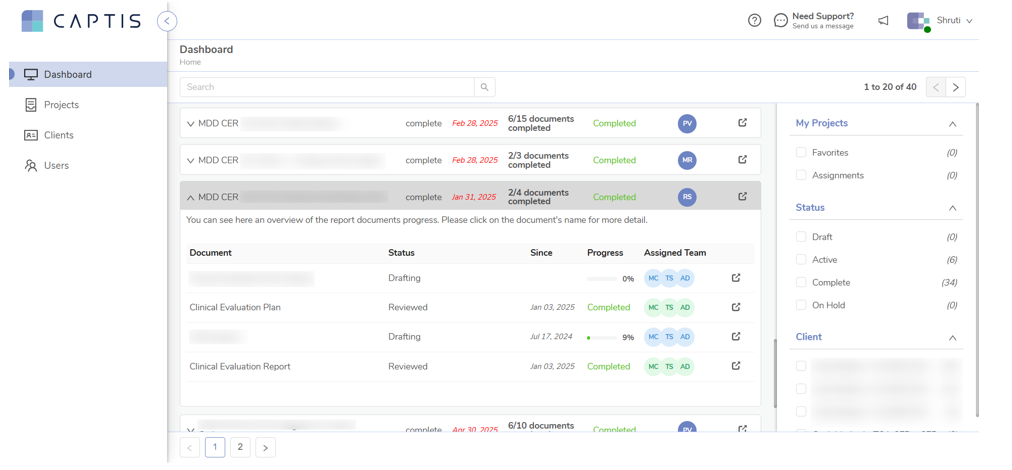
How Celegence Helps You Stay Ahead – Powered by CAPTIS®
[/fusion_title][fusion_text columns=”” column_min_width=”” column_spacing=”” rule_style=”” rule_size=”” rule_color=”” hue=”” saturation=”” lightness=”” alpha=”” user_select=”” awb-switch-editor-focus=”” content_alignment_medium=”” content_alignment_small=”” content_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” fusion_font_family_text_font=”” fusion_font_variant_text_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” logics=””]
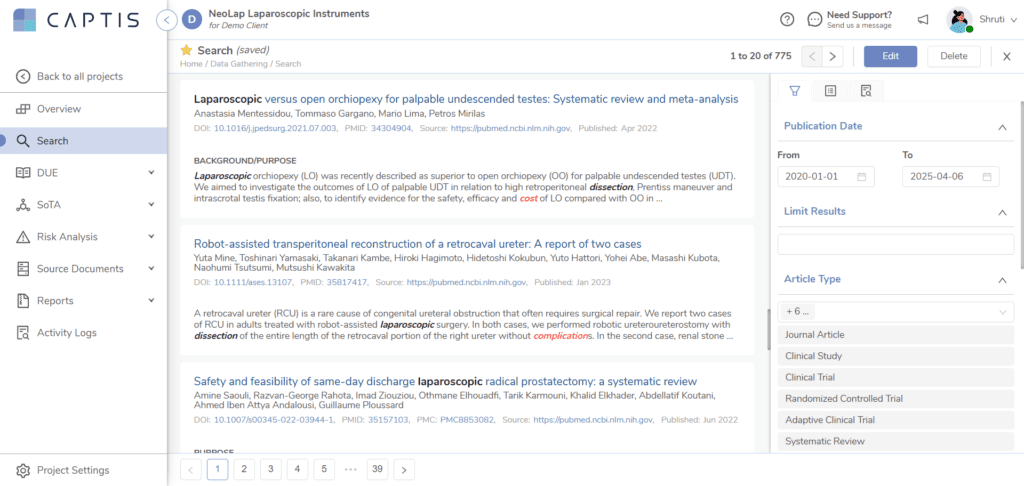
At Celegence, we understand the weight clinical evidence carried under EU MDR. That’s why our regulatory experts, clinical writers, and technologists work together to ease this burden for device manufacturers.
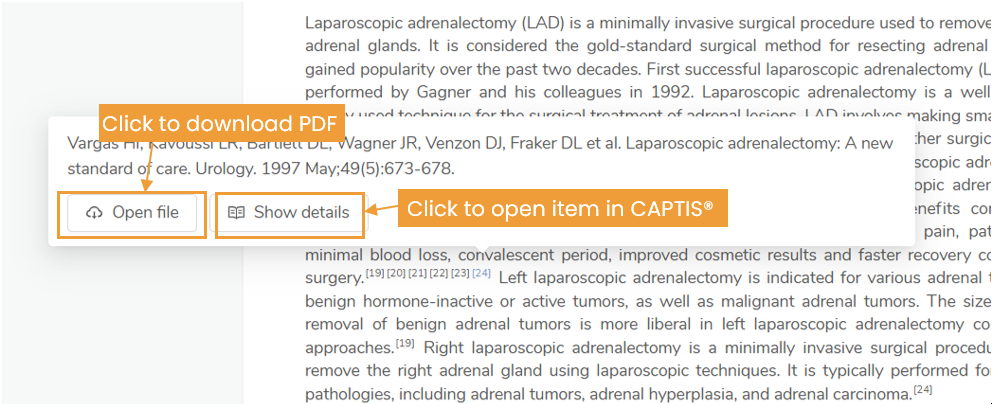
Our proprietary platform, CAPTIS®, supports every step of the clinical evidence development process:
-
- AI-Powered Systematic Literature Reviews – CAPTIS® uses intelligent algorithms to extract relevant data quickly and accurately, reducing manual effort and review cycles.
- Automated Data Consistency Checks – CAPTIS® ensures clinical data across documents is aligned, flagging inconsistencies before they’re submitted.
- Advanced Search & Data Retrieval – From real-time adverse event databases to journal articles, CAPTIS® connects you to the most relevant sources in moments.
- Audit-Ready Documentation – The platform generates clear, traceable reports that meet the expectations of Notified Bodies.
- Collaboration Tools – Clinical, regulatory, and quality teams can work together within CAPTIS® to maintain alignment across documentation.
Combined with our expert writing and consulting services, we deliver fully MDR-compliant CERs, PMCF plans, and literature reviews—all with quick turnaround time.
[/fusion_text][fusion_title title_type=”text” marquee_direction=”left” marquee_speed=”15000″ rotation_effect=”bounceIn” display_time=”1200″ highlight_effect=”circle” loop_animation=”off” highlight_width=”9″ highlight_top_margin=”0″ before_text=”” rotation_text=”” highlight_text=”” after_text=”” awb-switch-editor-focus=”” title_link=”off” link_url=”” link_target=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” content_align_medium=”” content_align_small=”” content_align=”left” size=”4″ animated_font_size=”” fusion_font_family_title_font=”” fusion_font_variant_title_font=”” font_size=”20px” line_height=”” letter_spacing=”” text_transform=”” text_color=”” hue=”” saturation=”” lightness=”” alpha=”” animated_text_color=”” text_shadow=”no” text_shadow_vertical=”” text_shadow_horizontal=”” text_shadow_blur=”0″ text_shadow_color=”” text_stroke=”no” text_stroke_size=”1″ text_stroke_color=”” text_overflow=”none” margin_top_medium=”” margin_right_medium=”” margin_bottom_medium=”” margin_left_medium=”” margin_top_small=”” margin_right_small=”” margin_bottom_small=”” margin_left_small=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” margin_top_mobile=”” margin_bottom_mobile=”” gradient_font=”no” gradient_start_color=”” gradient_end_color=”” gradient_start_position=”0″ gradient_end_position=”100″ gradient_type=”linear” radial_direction=”center center” linear_angle=”180″ highlight_color=”” style_type=”default” sep_color=”” link_color=”” link_hover_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=””]
Partner With Celegence
[/fusion_title][fusion_text columns=”” column_min_width=”” column_spacing=”” rule_style=”” rule_size=”” rule_color=”” hue=”” saturation=”” lightness=”” alpha=”” user_select=”” awb-switch-editor-focus=”” content_alignment_medium=”” content_alignment_small=”” content_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” sticky_display=”normal,sticky” class=”” id=”” margin_top=”” margin_right=”” margin_bottom=”” margin_left=”” fusion_font_family_text_font=”” fusion_font_variant_text_font=”” font_size=”” line_height=”” letter_spacing=”” text_transform=”” text_color=”” animation_type=”” animation_direction=”left” animation_color=”” animation_speed=”0.3″ animation_delay=”0″ animation_offset=”” logics=””]
Clinical evidence under EU MDR doesn’t have to be a bottleneck. With the right partner—and the right tools—you can build submission-ready documentation that stands up to regulatory scrutiny.
Talk to our clinical evidence team today and reduce your compliance burden with confidence.
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]