Raw Material Compliance in Pharmaceuticals
EMA Upgrades xEVMPD Web Interface

19 Feb, 2026
EMA Upgrades xEVMPD Web Interface: What MAHs Need to Know Before EVWEB Retirement (May 2026)
Introduction
The European Medicines Agency (EMA) has announced the release of a new web interface for xEVMPD (Article 57). For Marketing Authorization Holders (MAHs) relying on EVWEB, this upgrade introduces operational changes and a defined transition timeline. Here’s what regulatory teams need to prepare for ahead of the May 2026 retirement date.
Marketing Authorization Holders (MAHs) placing medicinal products on the EU market are required to submit and maintain accurate product information in the Article 57 (xEVMPD) database in accordance with EU pharmacovigilance legislation. For organizations that do not use gateway connection submissions (through RIMS), the EMA web-based reporting tool remains the primary interface for fulfilling these obligations.
As xEVMPD data supports multiple EMA regulatory and pharmacovigilance activities (PLM Portal, IRIS, CTIS), uninterrupted access to the reporting environment is essential to maintain compliance and regulatory operations in the EU. The recent upgrade of the xEVMPD web interface represents an important operational change for MAHs and their affiliates currently relying on EVWEB.
Below is an outline of what the upgrade entails, key timelines, and the practical considerations regulatory teams should address during the transition period.
What is the upgrade about?
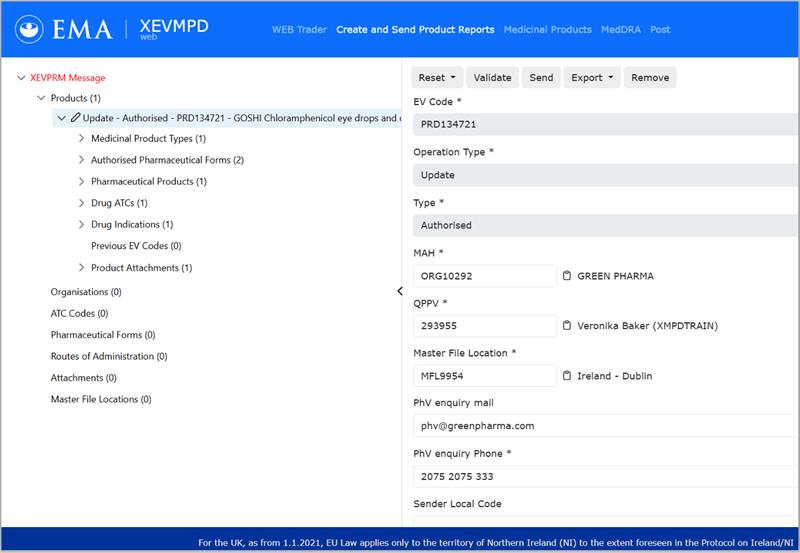
EMA has made available a new xEVMPD (xEVMPDweb) web tool to replace the use of EVWEB/Article 57 (Eudravigilance web reporting tool). The new tool comes with an improved user interface (UI) and the ability to send medicinal products information to EMA in any browser without the use of a browser extension or installation of supplementary software. Importantly, there are no changes to the submission process and compliance requirements of xEVMPD with this upgrade.
Why the upgrade?
While xEVMPD is planned to be decommissioned and replaced by IDMP, there are currently no defined timelines for this. Furthermore, many EMA systems rely on the information submitted through xEVMPD. Therefore, the reporting tool has been upgraded with a modern UI without the need to use IE tab (web-browser extension) and ActiveX installation, mitigating security and compliance risks, and improving usability. The new UI can be used in any browser, although the EMA recommends using Microsoft Edge or Google Chrome as the default browser to access xEVMPDweb.
How to access the new UI?
The new xEVMPDweb UI can be accessed with current EMA credentials. All current EVWEB users should be able to access the new tool without the need to request additional access roles. User roles are migrated to the new UI. The xEVMPDweb production environment is available in the Eudravigilance human restricted section. The xEVMPDweb XCOMP (test environment) is available through the following link (requires XCOMP user roles, if not already granted).
Figure 1 – xEVMPD new UI: Authorized product section
Transition timelines
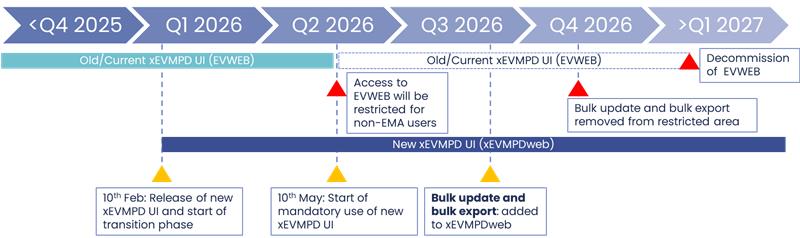
A transition period of 3 months started on February 10, 2026. Both EVWEB and xEVMPDweb will be available for MAHs and their affiliates during the transition period. The planned retirement date of EVWEB is the 10th of May 2026, at which point only the new xEVMPDweb UI will be available for MAHs and their affiliates for xEVMPD submissions.
Key Dates to Remember
- Transition start: 10 February 2026
- EVWEB retirement: 10 May 2026
- Bulk update tool integration expected: Q3 2026
Figure 2 – Transition timelines of EVWEB (old UI) and xEVMPDweb (new UI)
What does this mean for MAHs in practice?
- Test browser compatibility internally (EMA recommends Microsoft Edge or Google Chrome) and confirm there are no local IT restrictions impacting access.
- Update SOPs, work instructions, and training materials that reference EVWEB, IE tab extensions, or ActiveX components.
- Monitor the transition timeline closely and plan internal cut-over activities well before the end of the defined transition period.
- Troubleshooting and additional guidance to raise tickets to EMA ServiceDesk or reach out to our specialists for support.
Key Differences in the new UI
- ActiveX and IE tab no longer required for access to the web tool.
- Improved response time, browser compatibility, and readability.
- Menu and navigation :
- ‘Update’ and other XEVPRM operations in one drop down menu ‘Operations’
- Validation of imported XMLs: ‘Check for errors’ function when using ‘local import’ of XMLs
- All export options (RTF, XML, ZIP) are under ‘Export’
- Reset window and application options are under the ‘Reset’ menu
- WebTrader ‘Archived’ section was removed, users can query all acknowledgements and send XMLs using the advanced queries.
- Attachments added are to be uploaded immediately instead of after the submission is ‘sent’.
- No-pop up windows when exporting Excel files. Files are downloaded directly to the default download folder of the browser.
Final practical notes
While the EV post functionality is already part of the new UI, the bulk update tool is not. It remains accessible through the Eudravigilance restricted access area. The EMA expects the bulk update tool to be made available within the new UI in Q3 2026. For now, bulk update XMLs will be available in the WebTrader section of both EVWEB and xEVMPDweb (New UI). The bulk update process remains the same.
Additionally, a new entry for a user manual for xEVMPDweb is available in the Eudravigilance human restricted section under the ‘User Support’ section. For troubleshooting and additional guidance regarding the new xEVMPDweb UI, a service ticket should be raised at the EMA service desk.
Need Support During the xEVMPD Transition?
Celegence supports MAHs with operational xEVMPD data management, system transitions, and regulatory compliance activities across the EU.
Contact us at info@celegence.com to discuss how we can support your organization during the transition from EVWEB to xEVMPDweb.
Other Related Articles

06 Feb, 2026

16 Dec, 2025

02 Dec, 2025

11 Nov, 2025

04 Nov, 2025