Raw Material Compliance in Pharmaceuticals: Naming, Pharmacopeial Grade, and Safety Requirements
Understanding the Regulatory Considerations for Radiotherapeutics and Radiodiagnostics

03 Sep, 2025
Introduction to Radiotherapeutics and Radiodiagnostics
Scientific innovation and advances in ligand targeting have spurred the development of many different types of radiopharmaceuticals (RP) bound to biological molecules to target specific organs, tissues, or cells within the body with a wide range of clinical uses.
This is the first blog in the series “Understanding Radiotherapeutics and Radiodiagnostics”, where we explore their scientific foundations, applications, and the regulatory considerations critical for sponsors and manufacturers.
What Are Radiopharmaceuticals?
RPs (Radiopharmaceuticals) are drugs that contain, among other ingredients, radioactive forms of chemical elements (radioisotopes). Depending on the type of radiation that those radioisotopes produce, they can be used to diagnose or treat several medical conditions. Their applications range from imaging of many different organs, such as brain, heart, kidney and bone, to the treatment of cancer and hyperthyroidism. Radiopharmaceuticals are administered by orally or injection and can be monitored and analyzed with external medical devices and tests.
Structure of a Radiopharmaceutical
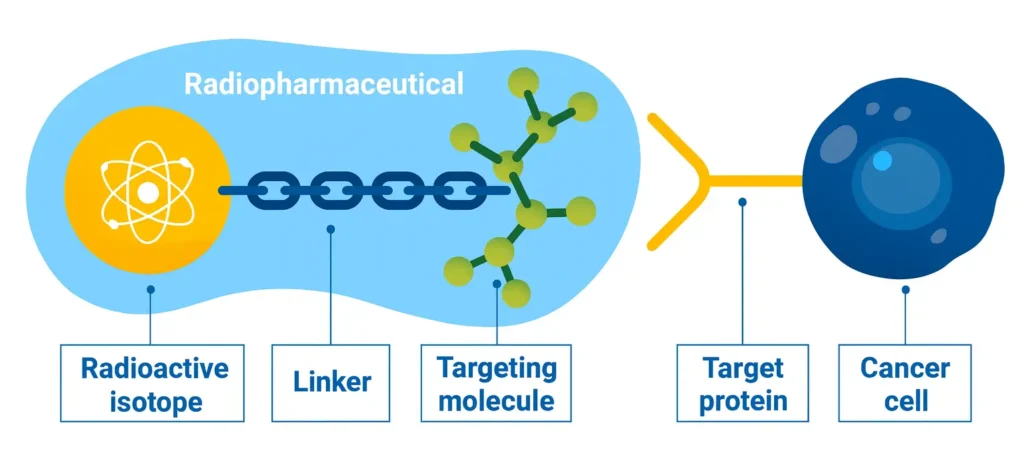
In addition to containing radioisotopes, radiopharmaceuticals contain molecules that target specific tissues or organs, e.g. oligosaccharides that home-in to tumors. These tumors consume more glucose than other parts of the body, thus ‘attracting the radioisotope-linked oligosaccharides thereby make the tumor visible (figure 1).
Figure 1: Structure of a Radiopharmaceutical
Radioactive drugs, or radiopharmaceuticals, combining a radioisotope with a tailored targeting molecule and a linker, ensuring a stable binding. This radiation could be gamma for diagnostic use, alpha or beta, for therapy. (Infographic: A. Vargas/IAEA)(1).
Growth and Development of Radiopharmaceuticals
The number of radiopharmaceuticals in clinical use is rapidly growing, thus increasing access to detail information on the characteristics of the different types of tumors and research is continuing to yield novel agents.
Two Main Classes of Radiopharmaceuticals
One of the basic challenges of RPs is that they are not, strictly speaking, a single class of agents, but two basic classes:
- Radiodiagnostics, which are used for scintigraphy imaging or measurement of biodistribution of biologically active substances.
- Radiotherapeutics, which incorporate radioisotopes to treat disease.
Radiodiagnostics
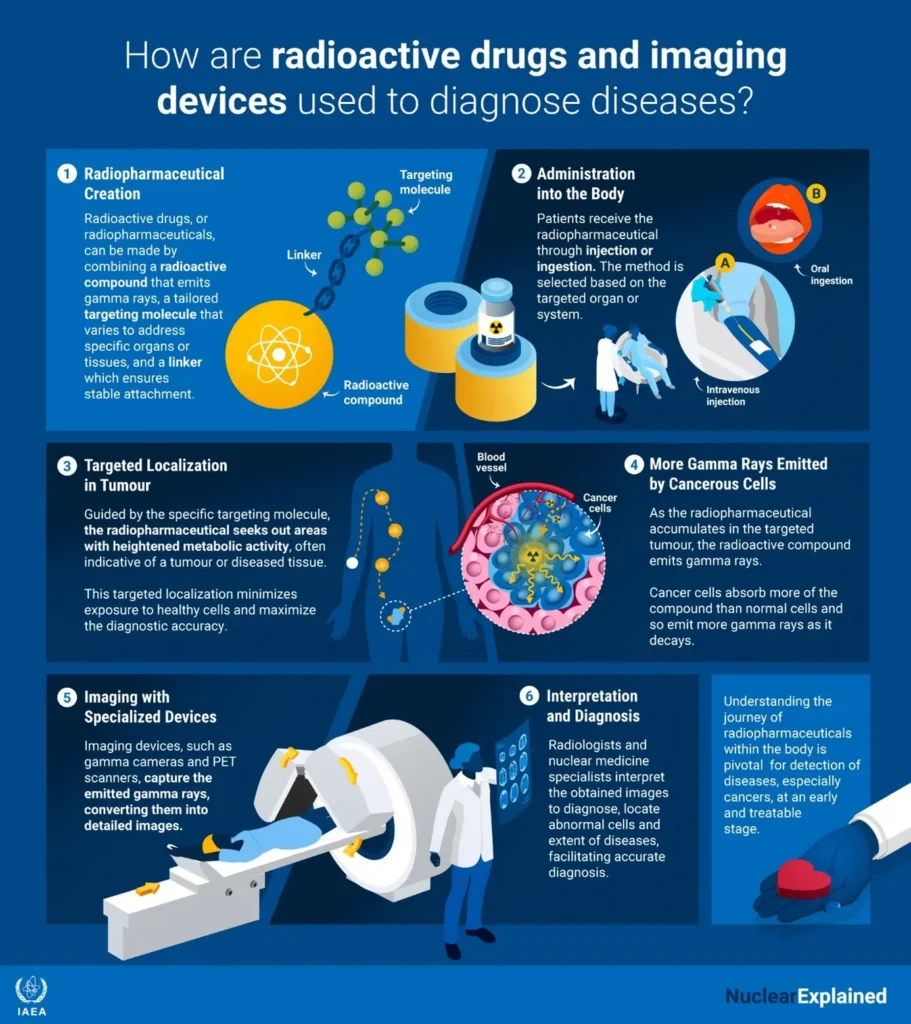
Radiodiagnostics are mainly used to facilitate diagnosis in the oncology, neurology, and cardiology settings. They incorporate radioactive tracers that emit gamma rays from within the body; the tracers are typically short-lived isotopes linked to chemical compounds that enable scrutiny of specific physiological processes (figure 2). Radiodiagnostics are used in medical procedures such as myocardial perfusion mapping, bone scans, and kidney scans. Additionally, laboratory techniques using radioactivity can detect underactive thyroids in newborn babies, making prompt treatment possible and saving many children from mental retardation. Radiodiagnostics are also used in biomedical research, pharmaceutical drug testing, metabolic research, and chemical reaction testing.
Figure 2: Use of Radiodiagnostics
Diagnostic radiopharmaceuticals emit gamma-radiation, which can penetrate the body and be detected by an external camera to produce images. (Graphic: A. Vargas/IAEA)(2)
Radiotherapeutics
Radiotherapeutics are commonly used to treat numerous types of cancers including metastatic bone cancer, prostate cancer, neuroendocrine tumors, leukemia, and lymphoma. Emerging radiotherapeutic applications include treatment of viral, fungal, and infectious diseases, which are increasingly resistant to current standards of care.
Common Features of Radiopharmaceuticals
A common feature to both classes is that many RP agents are prepared in small-batch quantities for use in exploratory trials, and may not be entered into a full development program leading to marketing authorization. Moreover, they are usually administered only once, or sometimes on a few occasions, and contain only small amounts of the active substances with a radionuclide attached to them. Oftentimes, such RPs do not show any measurable pharmacodynamic effect.
Regulatory Challenges for Radiopharmaceuticals
Developers of RPs are confronted with a regulatory environment that can be challenging to navigate, with health authorities struggling to keep up with the pace of innovation.
Nevertheless, as with other medicinal products, RPs should be governed by the same principles of efficacy and safety evaluation, both prior to use in human clinical trials as well as for an application for a marketing authorization.
Global Regulatory Frameworks
The regulatory pathway for RPs is thus defined by product type, intended use, and identified benefits and risks. Notably, different regions may have different regulatory frameworks and requirements for use of RPs (figure 3), and may even use different nomenclature: RPs are generally referred to as “radiotherapeutics” and “radiodiagnostics” in the United States and regulated by FDA and the Nuclear Regulatory Commission (NRC) In the European Union (EU) they are called “radiopharmaceuticals”, which are regulated as medicinal products.
Figure 3: Regulatory frameworks
How Celegence can help
Celegence’s team of subject matter experts in regulatory strategy for pharmaceuticals and medical devices ensures that both country-specific and market-wide regulatory requirements are met. We provide sponsors with tailored guidance and develop strategic roadmaps to maximize the efficiency of radiodiagnostics and radiotherapeutics in clinical trials. Most importantly, our experts act as on-the-ground partners, helping sponsors navigate the complexities of the regulatory landscape with confidence.
Contact us today at info@celegence.com to learn how our team can support your radiopharmaceutical programs with confidence and compliance.
References
Diagnostic radiopharmaceuticals. International Atomic Energy Agency. https://www.iaea.org/topics/diagnostic-radiopharmaceuticals. (accessed 03 FEB 2025)
What are Radiopharmaceuticals? International Atomic Energy Agency. https://www.iaea.org/newscenter/news/what-are-radiopharmaceuticals (accessed 03 FEB 2025)
Radioisotopes in medicine. World Nuclear Association; 2023. https://world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx
Other Related Articles

06 Feb, 2026

16 Dec, 2025

02 Dec, 2025