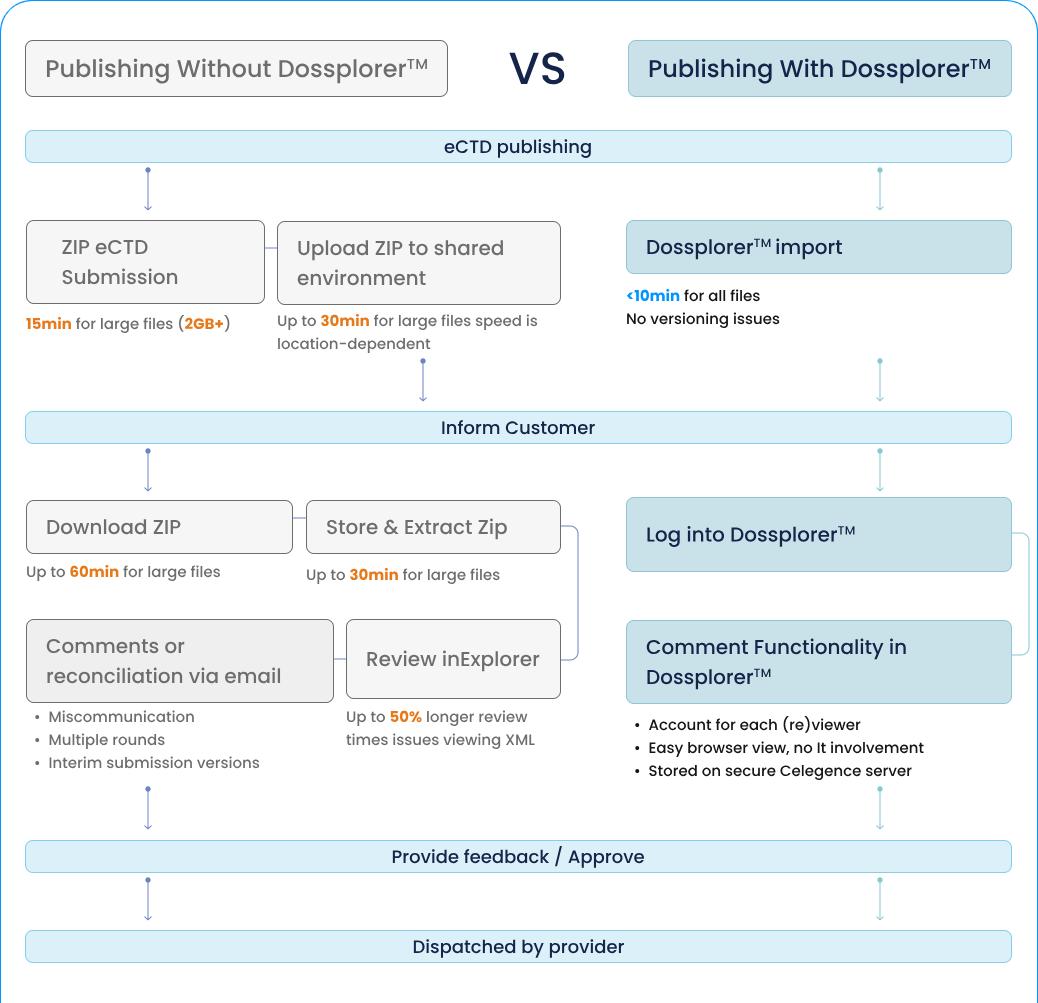
Common Challenges in DMF Submissions and How to Avoid Them
From document publishing automation to eCTD submissions and beyond, Celegence is your trusted partner for regulatory affairs excellence. Contact us to learn how we can help you achieve your compliance goals efficiently and cost-effectively.
"*" indicates required fields