Raw Material Compliance in Pharmaceuticals: Naming, Pharmacopeial Grade, and Safety Requirements
The Windsor Framework: A Game Changer for Medicines Regulation in Northern Ireland

05 Jun, 2025
Medicines in Northern Ireland
The Windsor Framework, in force since January 1, 2025, is an agreement between the United Kingdom (UK) and the European Union (EU) that to address the challenges of the Brexit transition, specifically regarding Northern Ireland. Its primary focus is the post-Brexit agreement to prevent a hard border with Ireland, while managing the UK’s exit from the EU. This was captured in the Northern Ireland Protocol, addressing trade issues between Northern Ireland and the rest of the UK, as well as the EU. The Windsor Framework now fine-tunes the Northern Ireland Protocol to make it more workable while keeping its core goal intact and helping to ensure the smooth movement of goods, including medicines, between Northern Ireland, the UK, and the EU. Thereby aiming to minimize the impact of Brexit on the medicinal product supply chain by ensuring continued access to medicines in Northern Ireland, maintaining regulatory alignment and avoiding new barriers to trade.
One of the most significant achievements of the Windsor Framework is the permanent guarantee that patients in Northern Ireland will have access to the same medicines as the rest of the UK. The MHRA now has the authority to license all medicines in Northern Ireland, including novel treatments that previously required approval through the European Medicines Agency (EMA).
This is the first blog in a series of three. Here we outline the key impacts on pharmaceutical companies and healthcare providers to adapt to the new requirements.
UK-Wide Licensing: A Unified Approach
Prior to the implementation of the Windsor Framework, different types of licenses were in use within the UK territory (Great Britain & Northern Ireland): the UK-wide, Great Britain only (GB-only), and Northern Ireland only (NI-only) licenses, each with their own prefix (Table 1).
With the implementation of the Windsor Framework, all medicines are licensed on a UK-wide basis. Centrally authorized products (approved by the European Commission) are no longer valid in Northern Ireland, and the MHRA has taken over authorization responsibilities.
Table 1: Types of licenses in the UK
| Authorization number prefix | Type of license | Situation after January 1, 2025 |
|---|---|---|
| PL | UK-wide MA | Issued for all new MAAs, except for NI-only |
| PLGB | Great Britain only MA | This type of license is automatically converted into a UK-wide license on January 1, 2025. No longer possible to apply for a new GB-only MA. |
| PLNI | Northern Ireland only MA (as approved via EMA) | Still possible to apply for a NI-only MA as part of the decentralized and mutual recognition procedures |
As of January 1,2025, all existing GB-only marketing authorizations were automatically converted to UK-wide licenses. Pending applications will also receive UK-wide validity without additional action from marketing authorization holders (MAHs). It is no longer possible to hold simultaneously a UK-wide license and a PLGB or PLNI for the same product. If an MAH holds a PLNI only and subsequently applies for a UK-wide authorization, the PLNI should be cancelled prior to the granting of the UK-wide marketing authorization.
Product Categorization
The changes that are introduced by the Windsor Framework apply to medicines that were previously within the scope of the EU centralized procedure. These products are now authorized under UK-wide MAs and no longer be limited in territorial scope as GB MAs. To clarify what rules apply to UK authorizations, all medicinal products that are licensed on a UK-wide basis (this includes both existing and new authorizations) are categorized as either ‘Category 1’ or ‘Category 2’ products.
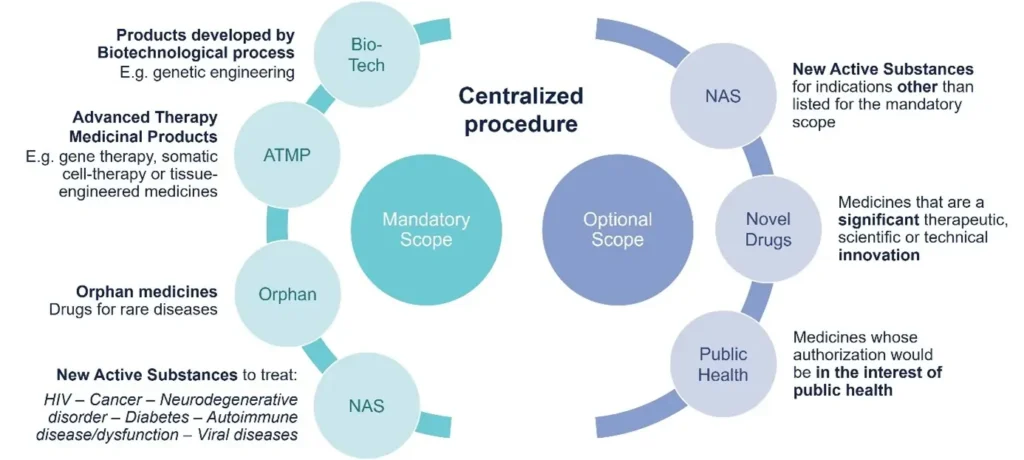
- Category 1: Products that fall within the mandatory or optional scope of the centralized procedure (see Figure 1) and will be authorized UK-wide under UK law.
Note that products authorized as conditional marketing authorizations also fall under Category 1 products (see also Table 2). - Category 2: All other products. These products will be authorized by the MHRA in accordance with UK law and applicable EU law on a UK-wide basis (see also Table 2).
Products that are authorized through generic, biosimilar, and hybrid applications will follow the same Windsor Framework category as their corresponding reference product.
Table 2: Overview of Category 1 and Category 2 medicinal products
| Category 1 | Category 2 | |
|---|---|---|
| Authorized Products | Authorized products in the EU through the centralized procedure and subsequently grandfathered at the time of EU Exit.
Authorized products within the mandatory scope of the centralized procedure authorized by MHRA since 1 January 2021. Authorized products with the optional scope of the centralized procedure authorized by MHRA since 1 January 2021. |
Authorized products that do not fall within the mandatory scope of the centralized procedure.
Authorized products that are eligible for the optional scope of the centralized procedure, but are authorized through national procedures in the EU, including MRP/DCP. Products authorized prior to the introduction of the centralized procedure. |
| New Applications | New applications for products that fall within the mandatory or optional scope of the centralized procedure. This includes products authorized as conditional marketing authorizations. |
New applications for products that do not fall within the mandatory or optional scope of the centralized procedure (i.e., not within the scope of Category 1). This will not include products authorized as conditional marketing authorizations. |
| Generic, Hybrid or Biosimilar Products | Generic, hybrid or biosimilar products of a Category 1 reference product. For already authorized products, this will apply regardless of whether the centralized procedure was used for the generic, hybrid or biosimilar application. |
Generic, hybrid or biosimilar products of a Category 2 reference product. |
Figure 1: Products in scope of the EU Centralized Procedure
Key Takeaways on Licensing Changes Under the Windsor Framework
The Windsor Framework significantly streamlines medicine regulation in Northern Ireland, ensuring that UK patients receive equal access to treatments. By transferring licensing authority to the MHRA the framework provides much-needed regulatory certainty for pharmaceutical companies. However, businesses must ensure compliance with the new requirements since January 2025.
For detailed guidance, visit the official MHRA Windsor Framework collection: MHRA Windsor Framework Guidance.
The next blog of this series will discuss the procedural impact of the changes introduced under the Windsor Framework.
At Celegence, we support pharmaceutical organizations with end-to-end UK regulatory strategy, submissions, and compliance services. Contact us at info@celegence.com to discuss how we can assist your team
Other Related Articles

06 Feb, 2026

16 Dec, 2025

02 Dec, 2025