How AI Is Transforming CER Development for Medical Devices & IVDs
Case Study: How a Global MedTech Firm Streamlined Regulatory Writing with CAPTIS® & Celegence

26 May, 2025
Executive Summary
Regulatory documentation projects often struggle with version control issues, manual processes, and fragmented review cycles. For one global medical device manufacturer, these pain points were slowing down their ability to deliver high-quality, timely submissions. By partnering with our expert medical writing team and using our AI-powered software platform CAPTIS®, they transformed the way they draft, review, and finalize regulatory reports. The result: faster turnaround times, reduced errors, and a more collaborative experience.
The Challenge
Before working with us, the client relied on traditional drafting workflows using Microsoft Word, shared over email. With multiple writers and reviewers involved, the process was fraught with:
- Frequent document crashes
- Formatting inconsistencies across Word versions
- Time-consuming manual updates of citations, abbreviations, and references
- Confusion from version mismanagement
- Delayed feedback due to sequential (not parallel) review workflows
Despite having competent internal reviewers, they lacked the tools and processes to streamline document development. Collaboration was siloed, and reviews were often delayed or duplicated.
Our Solution
By combining our experienced medical writing team with our proprietary software CAPTIS®, Celegence helped the client transition to a faster, structured, and AI-augmented documentation process.
Key workflow upgrades included:
- Centralized Document Management: All literature, documents, source files, and review versions live in one place—with real-time version control.
- Real-Time Collaboration: Multiple users can draft or review simultaneously, without crashes or version confusion.
- Automated Formatting & Styling: Templates ensure consistency, removing hours of manual formatting.
- Integrated Data Dictionary: Updates to a term auto-propagate across all documents, ensuring consistent language and definitions.
- Abbreviation Table Automation: Abbreviations and their full forms are auto populated, improving clarity and compliance.
- Citations and Reference Management: Citations are easy to create and the bibliography inserted automatically, with a structured reference pack compiled for each report. Reviewers can open PDFs of references with just a click.
Figure 1: One-click access to reference PDFs directly from the document for seamless verification during review.
- Smart Navigation via Content Links: Built-in navigation and content links make jumping between document sections and referenced documents seamless.
Figure 2: Seamless navigation through clickable content links that connect document sections and referenced source information
- AI-Assisted Evidence Gathering: Our built-in Copilot offers literature content summarization and Q&A capabilities for faster insights and validation.
Figure 3: AI-Assisted Evidence Gathering
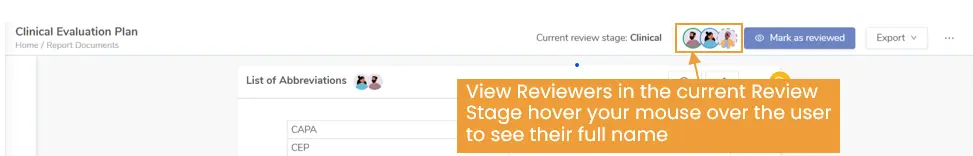
- Review Stage Controls: Users can tag team members, lock sections, and stage reviews, creating transparency and accountability.
Figure 4: View designated reviewers for each review stage
- Automated Review Initiation Notifications: All reviewers are automatically notified when their designated review stage begins, eliminating manual follow-ups and delays.
Figure 5: Automated review notification alert sent to designated reviewers at the start of their review stage
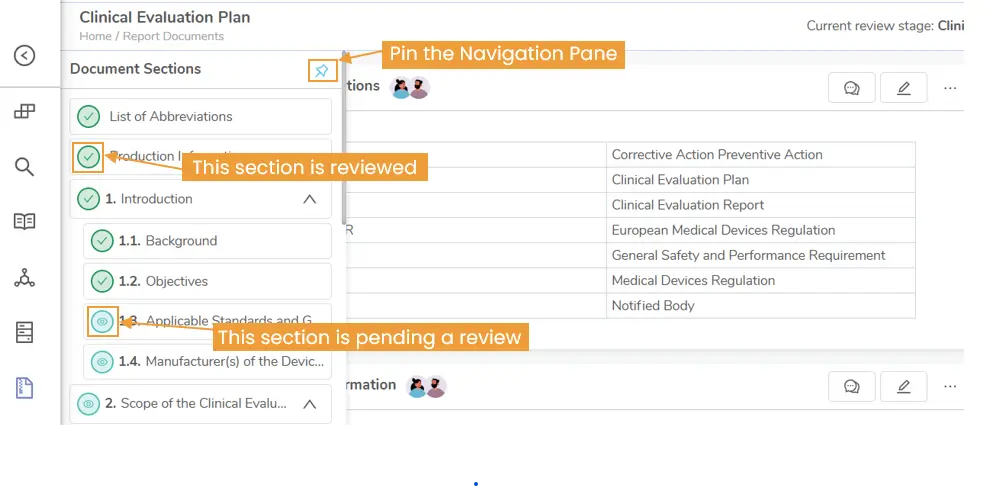
- Review Progress Tracking: Reviewers can instantly see which sections they’ve reviewed and what’s still pending, making it easy to pick up where they left off.
Figure 6: Clearly see which sections are reviewed and which are pending. Navigate effortlessly using the section panel
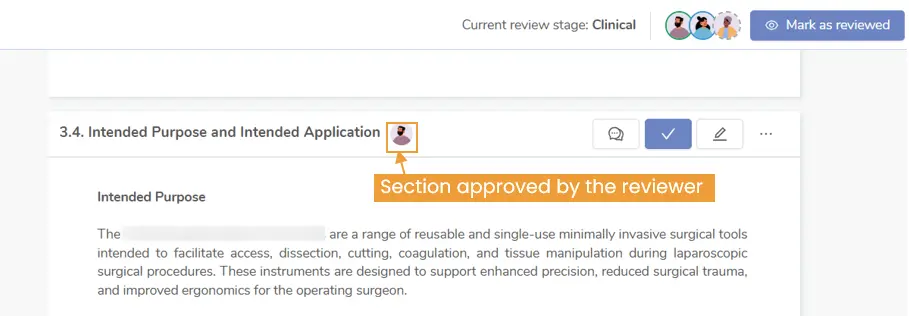
- Reviewer Approvals Visibility: Client reviewers can quickly view which sections have been approved by other reviewers to avoid duplication and streamline collaboration.
Figure 7: Easily track approved sections to enhance collaboration and prevent duplication
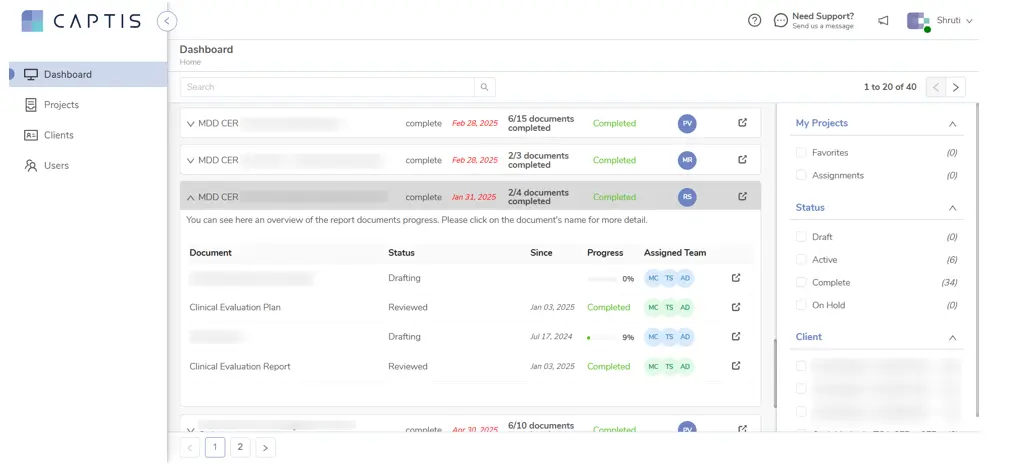
- Integrated Dashboard: A powerful dashboard allows the client to track the progress of multiple projects in real-time, with clear indicators of where each document stood in the drafting and review cycle, and a quick look at which reviewers had started or completed their tasks.
Figure 8: Track real-time progress across projects with a clear view of drafting stages, review status, and writer/reviewer activity—all in one place
Outcomes and Benefits
Since implementing CAPTIS and collaborating with Celegence’s regulatory medical writing team, the client has experienced measurable improvements:
- Faster Turnaround Times: Document drafting and review cycles were significantly shortened through real-time collaboration, automation, and AI support—enabling quicker report finalization and submission.
- Full Workflow Transparency & Control: An integrated dashboard and structured review stages gave the client complete visibility into document progress, reviewer status, and project timelines across multiple reports.
- Streamlined Collaboration & Reviews: Features like tagging, comments, shared access, and reviewer-specific views reduced communication gaps, avoided duplication, and made it easy for teams to coordinate and review efficiently.
- Greater Consistency & Compliance: Centralized document storage, an auto-updating data dictionary, citation tools, and version control ensured accurate, consistent, and audit-ready content across all deliverables.
- Smart Automation & AI Assistance: Built-in tools for abbreviation tables, reference management, review stage notifications, and AI-driven literature summarization and Q&A significantly reduced manual work and improved productivity.
- Flexibility & Scalability: The system adapted to project changes—like updated terminology or literature search strategies—without disrupting ongoing work, ensuring smooth execution even in dynamic regulatory environments.
Conclusion
Working with Celegence isn’t just about regulatory expertise—it’s about transforming how regulatory documents are created and reviewed. By pairing our deep domain knowledge with a powerful literature review and documentation platform, we helped a major MedTech player gain efficiency, transparency, and confidence in their submissions.
Want to streamline your next regulatory writing project?
Work with us to get not just expert writers – but a smarter, faster way to collaborate.
Other Related Articles

08 Jan, 2026

23 Dec, 2025

08 Dec, 2025

20 Nov, 2025

11 Nov, 2025