Regulatory dossiers contain comprehensive and detailed information about pharmaceutical products. Since they serve as the primary source of truth for regulatory agencies to assess safety, quality, and efficacy, they play a crucial role in the regulatory approval process.
This valuable information, however, remains hidden from the users. In their day-to-day operations they often rely on various systems to access regulatory content. The main challenge lays in the fact that the data and the documents are in different systems and there is a disconnect between them.
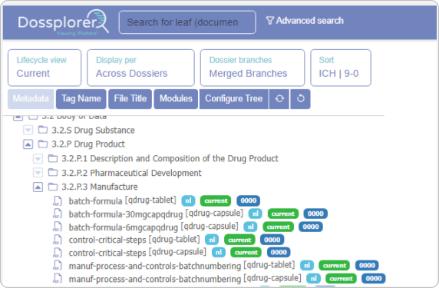
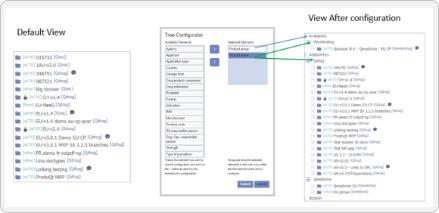
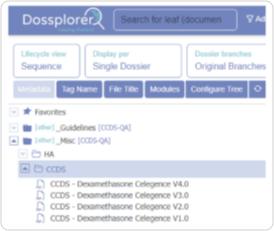
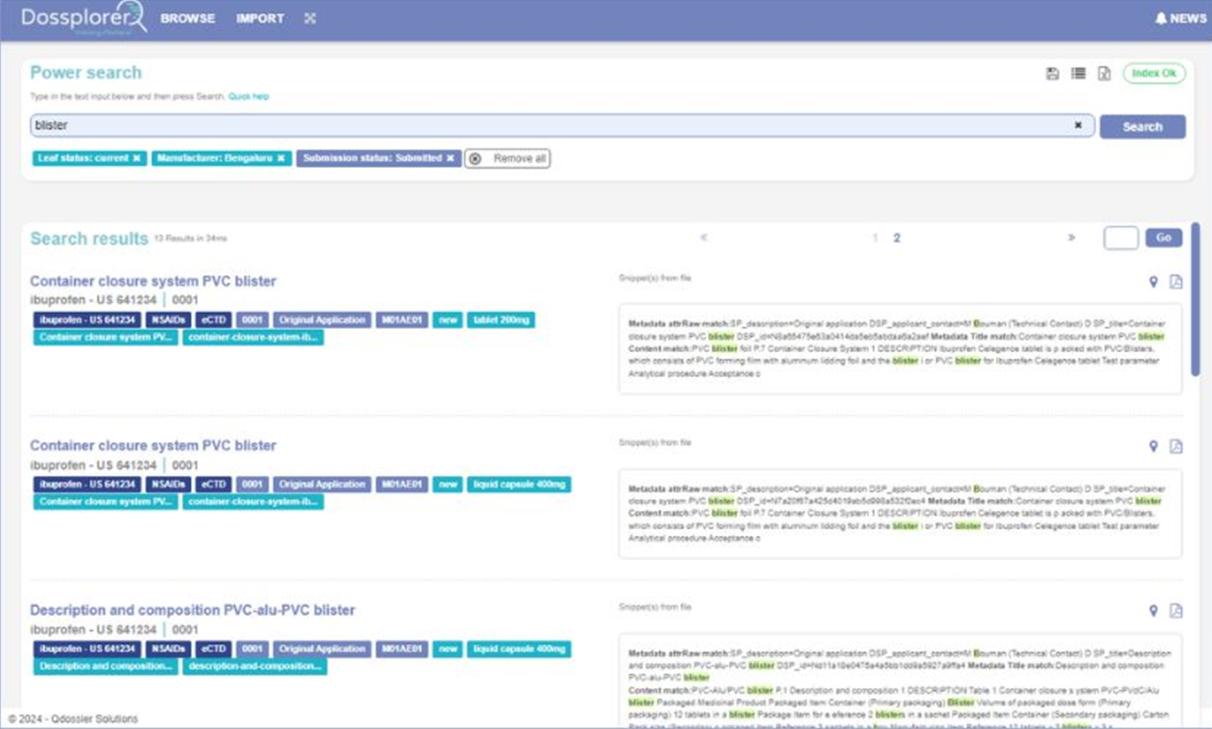
Dossplorer™ saves dozens of hours for regulatory teams by eliminating process inefficiencies, ZIP file downloads and extractions, and multiple review steps.