Roll-out of the In Vitro Diagnostics Medical Device Regulation in Europe (EU IVDR)
The EU IVDR regulation entered into force and was adopted on May 26th, 2017. Following a transition period of 5 years, it is all set to enter into application on May 26th, 2022. The regulation introduced several stringent regulatory requirements for IVD manufacturers and other economic operators. At the same time, it has intensified the role of the notified bodies. It also brings in the need of additional infrastructure such as EU reference laboratories for testing high risk devices, and the requirement of setting up of expert panels whose opinion is required during certification. In short, the change from the IVD Directive to the IVD regulation is a drastic one for all stakeholders and even requires certain infrastructure to be in place for its implementation.
IVDR Roll-out Current Status
With the application deadline approaching, the current situation shows that this infrastructure is not quite ready. It is estimated that around 70% of clinical decisions are made using IVDs. Around 80% of IVDs require notified body involvement under the IVDR. Currently, there are only six notified bodies which have been designated for carrying out IVDR assessments for the entire IVD industry and these are established in only three countries (Germany, France, and the Netherlands). Firstly, apart from this small number, they have limited capacities in terms of resources. Secondly, the EU Reference Labs required for the testing of Class D IVDs are yet to be designated. Thirdly, the EUDAMED implementation also experienced delays, but some modules are functional as of now.
On the other hand, the COVID-19 pandemic and the subsequent health care crisis brought in unprecedented challenges for the implementation of the IVDR since late 2019. The manufacturing industry has been badly impacted with several countries under lock down. To address the challenges put up by the pandemic, the manufacturers also had to redeploy financial resources to other priority areas. They ran into several challenges from facing shortages for raw material and other resources to working under constraints. In some cases, the manufacturers shifted priorities from IVDR transition to other activities like actually manufacturing COVID-19 kits.
The checklist highlights all of the documentation that you will need in place for certification of your IVD device and will serve as a guide to help you achieve ongoing compliance. In conjunction with this checklist, we are also able to provide you with bespoke strategies to bring your business up to speed. We are currently working with businesses from the United States, India, and throughout Europe to ensure that they are ready for the deadline in May of 2022.
List of IVDR Notified Bodies
To understand market readiness for IVDR implementation, the European Commission collected data during the first half of 2021 which showed that Member States, health institutions, notified bodies and economic operators will not be in a position to ensure the proper implementation and application of the Regulation from 26 May 2022.
To begin with, since there are just six notified bodies to cater to the entire IVD industry as of Dec 2021, there is a bottleneck to certifying devices.
Ref: European Commission
| Country | Regulatory body | Regulation |
|---|---|---|
| NB 2797 | BSI Group The Netherlands B.V. | Netherlands |
| NB 0344 | DEKRA Certification B.V. | Netherlands |
| NB 0124 | DEKRA Certification GmbH | Germany |
| NB 0197 | TÜV Rheinland LGA Products GmbH | Germany |
| NB 0123 | TÜV SÜD Product Service GmbH Zertifizierstellen | Germany |
| NB 0459 | GMED SAS | France |
Table 1: Notified Bodies for EU IVDR.
Consequently, many of the self-certified IVDs under the IVDD which are up-classified under the IVDR, will soon be off the European market. As a result, a huge lacuna will be created in the diagnostic capabilities in Europe. This could lead to a healthcare crisis arising from the unavailability of a large number of IVDs on the market, for both the health institutions and the public.

IVDR Amendment Proposal
The European Commission put up a
“Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Regulation (EU) 2017/746 as regards transitional provisions for certain in vitro diagnostic medical devices and deferred application of requirements for in-house devices”
on Oct 14th, 2021, to address this challenging situation. The proposal suggested a phase-wise roll out of the IVDR, by amending Article 110 of the IVDR, which should be based on the risk class of the device. This would lead to extending transition timelines such that devices such as HIV tests, pregnancy tests, or SARS-CoV-2 tests currently under IVDD can remain on the market for a longer period, circumventing a healthcare crisis in Europe. This would also give the notified bodies sufficient time to carry out conformity assessments. The EC would also get time for putting in place the necessary infrastructure for implementation.
Adoption of the IVDR Proposal and What it Means
This proposal has now been adopted by the European Parliament and council in Dec. 2021. The adoption of this proposal ensures that the supply of essential healthcare products is not interrupted.
Let us now understand what the adopted proposal brings in for IVD manufacturers. The key points are as follows:
- The amendment does not change any of the requirements of the IVDR.
- The timelines are to be extended for those devices under IVDD which are up-classified under IVDR. This spreads the number of devices undergoing conformity assessments over a longer period, thus addressing the issue of availability of notified bodies. At the same time it prioritizes high risk devices.
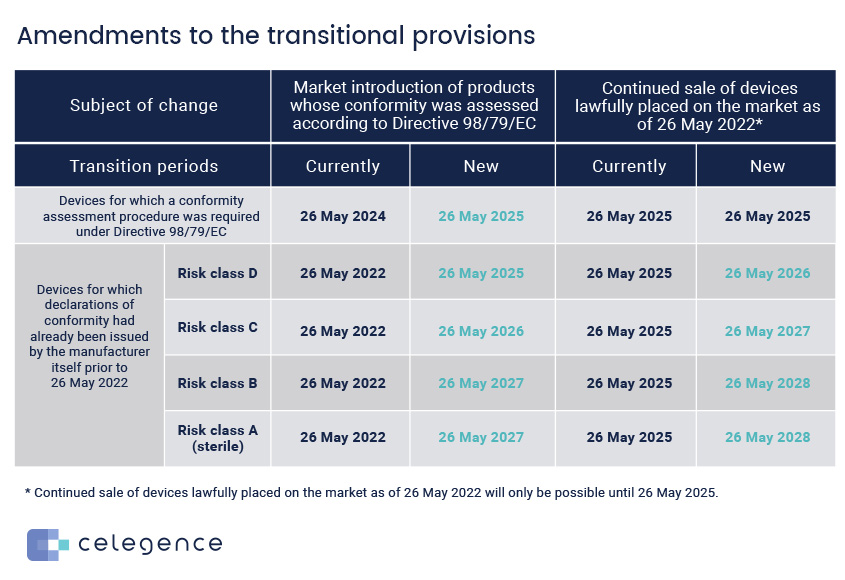
- Class D devices(highest risk) – Transition period extended until May 2025
- Class C devices(high to moderate risk) – Transition period extended until May 2026
- Class B and A sterile(moderate to low risk) – Transition period extended until May 2027
- Class A Non-sterile – There is no change for these devices and their manufacturers will have to meet the May 2022 deadline. (Important)
Note: Most instrument manufacturers come under this category. For them there is no change.
- The original IVDR gave a “grace period” until May 2024 for some devices that were already CE marked under the IVDD. This grace period is now to be extended to May 2025, provided, there are no significant changes to the design and intended purposes of these devices. If there are significant changes they need to apply for the IVDR.
- The IVDR had made some “sell-off provisions” (art. 110 par. 4 IVDR), which limits the timeframe an IVDD compliant device can be placed on the market or put into service. The provisions are for devices already in the supply chain and can actually be made available to hospitals, for example (reach the final user).
These key dates are:
-
- May 2025 – if the device was placed on the market prior to May 2022. After May 27th, 2025, these devices may not be available/put into service. If such devices are still within the supply chain by this date, they are no longer considered marketable devices.
- Devices placed on the market after May 2022 benefit from a sell-off provision as follows:
- Class D – until May 2026
- Class C – until May 2027
- Class B and A sterile – until May 2028
- The proposal also considers in-house devices or laboratory designed tests made by health institutions (i.e. LDTs). The EC proposes to defer the application of most of the conditions to be met by health institutions making in-house devices by two years until May 26th, 2024.
Two Key Considerations – The Bottom Line
- After May 2022, all devices on the European market still have to comply with the IVDR requirements related to PMS, vigilance, device registration and registration of economic operators.
- For new IVDs the Regulation (EU) 2017/746 will apply in full from May 26th, 2022.
The checklist highlights all of the documentation that you will need in place for certification of your IVD device and will serve as a guide to help you achieve ongoing compliance. In conjunction with this checklist, we are also able to provide you with bespoke strategies to bring your business up to speed. We are currently working with businesses from the United States, India, and throughout Europe to ensure that they are ready for the deadline in May of 2022.