Contact Lens Manufacturers: European Commission Amendments on UDI Requirements
The European Commission has made significant amendments to Regulation (EU) 2017/745, which addresses medical devices, specifically focusing on the Unique Device Identification (UDI) system. This system plays a crucial role in ensuring the identification and traceability of medical devices in the market. Recent collaborations with industry stakeholders have resulted in specific changes regarding assigning UDI-DIs (Device Identifiers) to contact lenses.
Delegated Regulation for Contact Lenses
On 10 July 2023, the Commission introduced a Delegated Regulation, enabling the grouping of standard contact lenses and made-to-order contact lenses with the same design parameters under a single Master UDI device identifier (UDI-DI). This modification aims to alleviate administrative burdens for manufacturers compared to other jurisdictions’ practices.
UDI for Contact Lenses – Problem and Solution
Currently, each variant of contact lenses is assigned a unique UDI-DI, leading to an overwhelming number of identifiers in the European database on medical devices (EUDAMED). This excessive assignment is disproportionate to the safety risk associated with contact lenses. To address this issue, the European Commission proposes a Master UDI-DI approach. Under this system, similar contact lenses with the same clinical and design parameters will be grouped together and identified with a Master UDI-DI. When there is a change in the combination of design parameters, a new Master UDI-DI will be required.
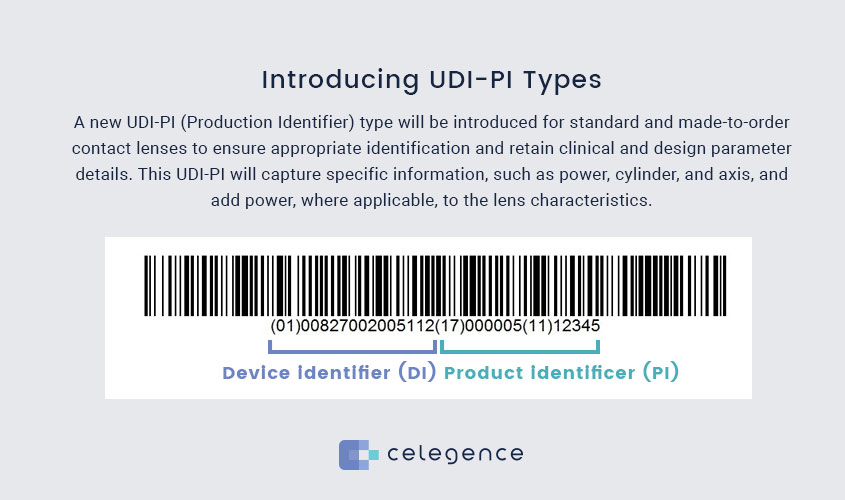
Introducing UDI-PI Types
A new UDI-PI (Production Identifier) type will be introduced for standard and made-to-order contact lenses to ensure appropriate identification and retain clinical and design parameter details. This UDI-PI will capture specific information, such as power, cylinder, and axis, and add power, where applicable, to the lens characteristics.
Implementation and Compliance
Economic operators must change their internal systems and adapt technologies for printing and scanning UDI carriers to comply with the new Regulation. The application of this Regulation will be deferred, with a two-year transition period for manufacturers to adjust their processes. However, manufacturers may voluntarily start assigning Master UDI-DIs and UDI-PIs in accordance with the amended Regulation before the official implementation date.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
Key Takeaway for Contact Lens Manufacturers
The recent amendments to the EU Medical Device Regulation (MDR) introduce a significant change in the Unique Device Identification (UDI) system, particularly for contact lenses. Manufacturers should pay close attention to the following key takeaway:
- Master UDI-DI for Contact Lenses: Manufacturers now have the option to assign a Master UDI-DI to groups of highly individualized devices with similar clinically relevant parameters. This approach streamlines UDI reporting in the European Database on Medical Devices (EUDAMED) and enhances traceability.
- Simplifying UDI Reporting: The introduction of the “Master UDI-DI” concept aims to simplify the UDI system, making it more efficient for contact lenses. By grouping similar devices, manufacturers can avoid assigning multiple UDI-DIs for slight variations in clinical parameters.
- Adhering to UDI Changes: Manufacturers should be prepared to implement the new Master UDI-DI system once the Regulation comes into effect. They can start assigning Master UDI-DIs in line with the amended Regulation (EU) 2017/745 before the official application date.
- Implementation and Compliance: Economic operators must change their internal systems and adapt technologies for printing and scanning UDI carriers to comply with the new Regulation. The application of the Regulation will be deferred, and it will take effect on the twentieth day following its publication in the Official Journal of the European Union. Manufacturers can assign a Master UDI-DI in accordance with the amended Regulation before the official implementation date.
- Potential Expansion: Although the primary focus is currently on contact lenses, the Master UDI-DI concept may extend to other highly individualized devices. Manufacturers should stay informed about any further developments in this regard.
By embracing these changes and proactively adapting their practices, manufacturers can ensure smooth compliance with the UDI requirements for contact lenses, improving efficiency and regulatory adherence in the European market.

UDI Changes for Contact Lens Manufacturers Conclusions
The proposed changes to the UDI system for contact lenses aim to enhance efficiency and reduce redundancies in the European medical device database. By adopting the Master UDI-DI approach and introducing new UDI-PI types, the identification and traceability of contact lenses will be improved while ensuring patient safety and regulatory compliance. Manufacturers are encouraged to adapt their practices per the amended Regulation to streamline the UDI process for contact lenses effectively.
Reference:
- Amending Regulation (EU) 2017/745 of the European Parliament and of the Council, as regards the assignment of Unique Device Identifiers for contact lenses (Link: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13299-Medical-devices-single-identifier-for-similar-highly-individualised-devices_en)
- Commission Implementing Decision (EU) 2019/939 of 6 June 2019 designating issuing entities designated to operate a system for the assignment of Unique Device Identifiers (UDIs) in the field of medical devices (Link: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019D0939)